

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535166 (WO2022013684, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509922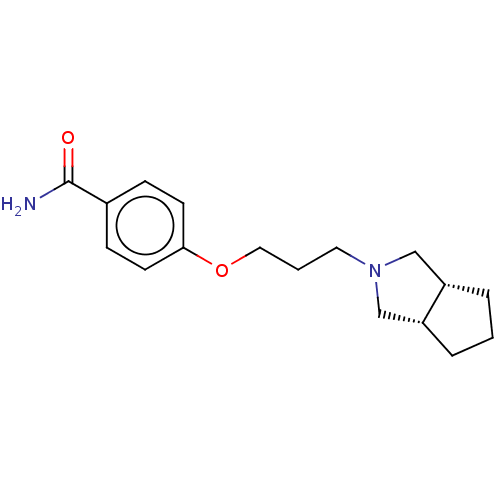 (CHEMBL4575319) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535159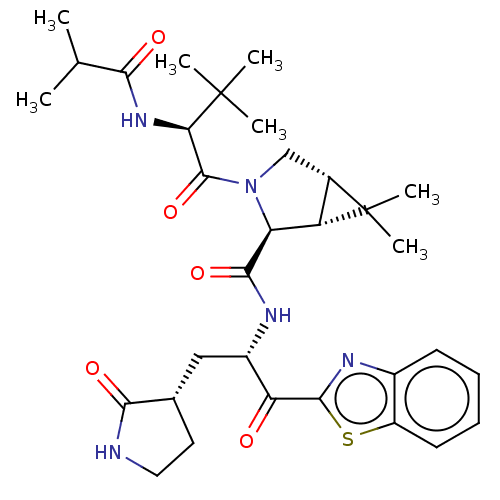 (WO2022013684, Example 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535160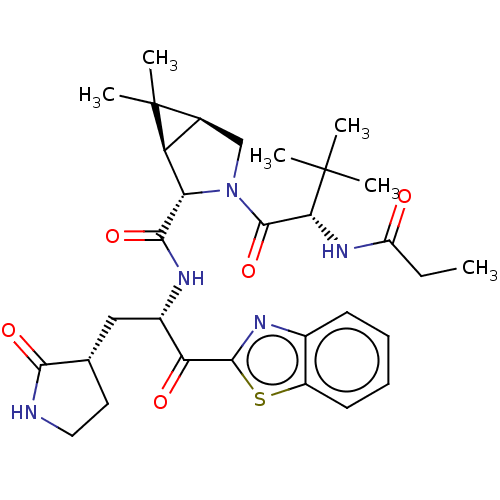 (WO2022013684, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509934 (CHEMBL4557586) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535126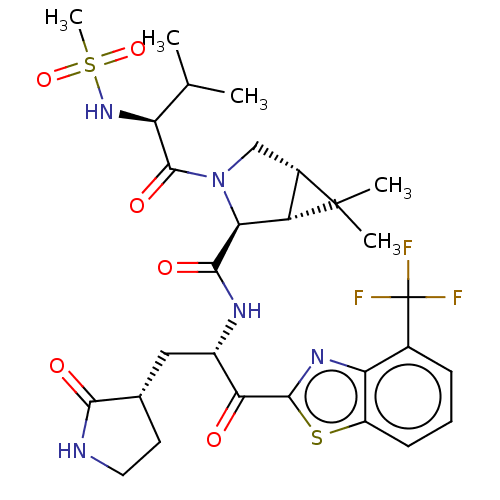 ((1R,2S,5S)-6,6-Dimethyl-3-[N-(methylsulfonyl)-L-va...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 7.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535168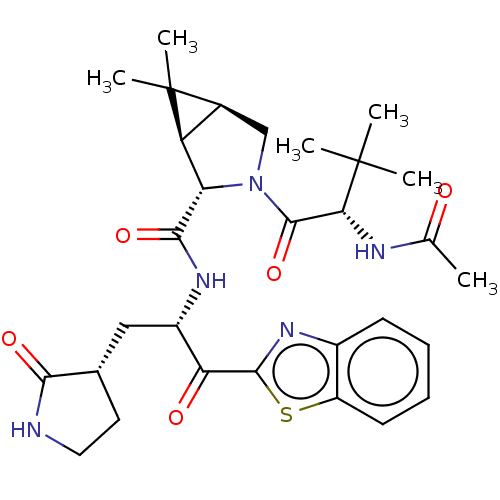 (WO2022013684, Example 19) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 9.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535164 (WO2022013684, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535123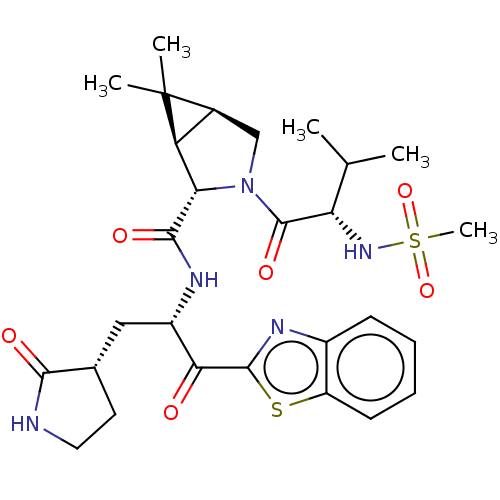 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535143 (WO2022013684, Example 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535129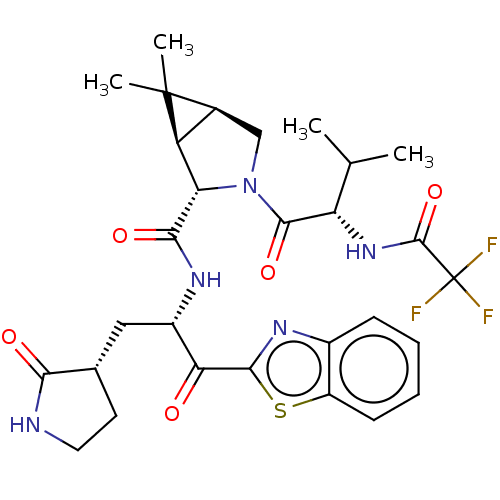 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | WIPO WO2022013684 | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535075 ((6S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo-3-[(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535141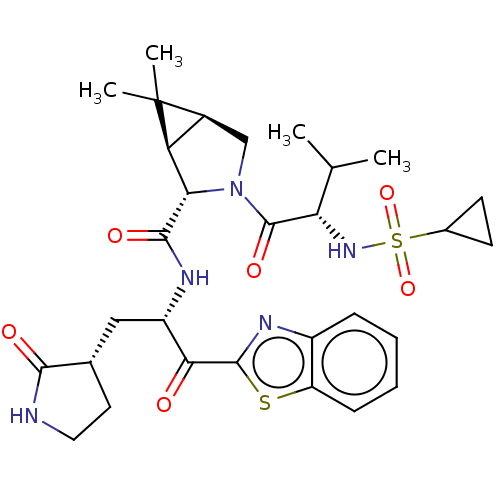 (WO2022013684, Example 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509933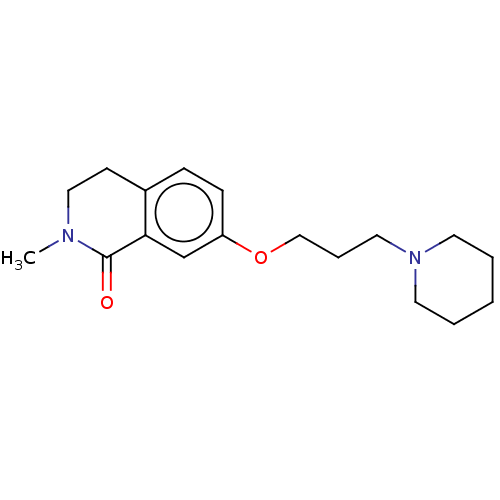 (CHEMBL4475658) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509924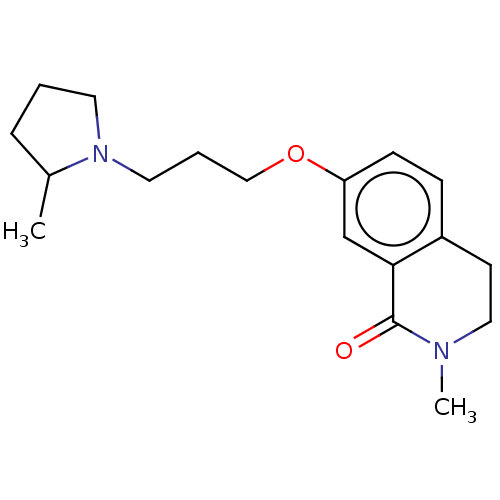 (CHEMBL4532574) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509932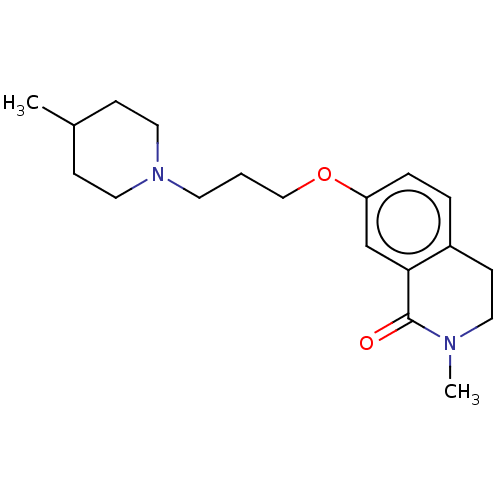 (CHEMBL4526723) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535145 (WO2022013684, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509928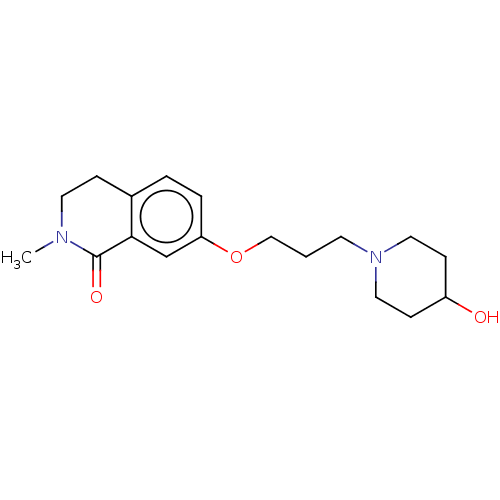 (CHEMBL4583183) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509923 (CHEMBL4456092) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509924 (CHEMBL4532574) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535165 (WO2022013684, Example 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509929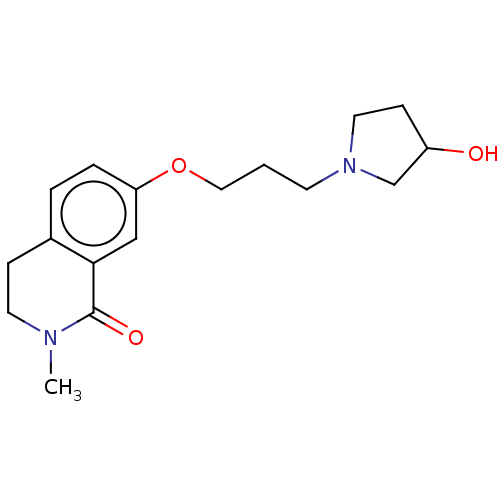 (CHEMBL4570965) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535130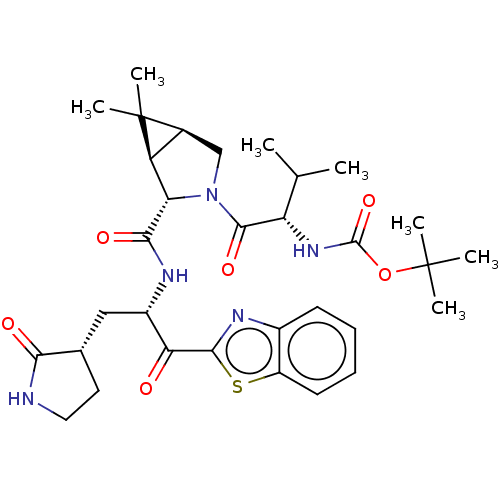 (WO2022013684, Example 6 | tert-Butyl {(2S)-1-[(1R,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535146 (WO2022013684, Example 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509936 (CHEMBL4461154) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509923 (CHEMBL4456092) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 169 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509921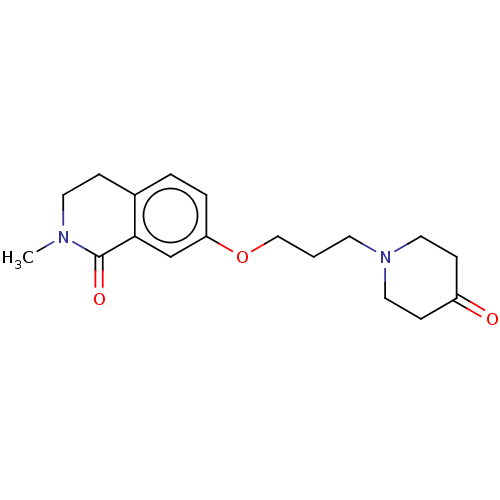 (CHEMBL4474007) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509933 (CHEMBL4475658) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535122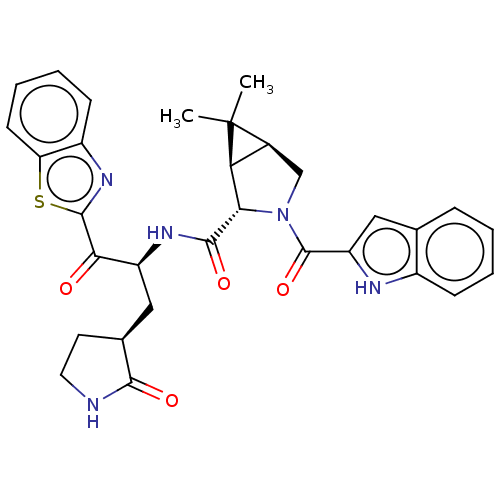 ((1R,2S,5S)-N-{(2S)-1-(1,3-Benzothiazol-2-yl)-1-oxo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509925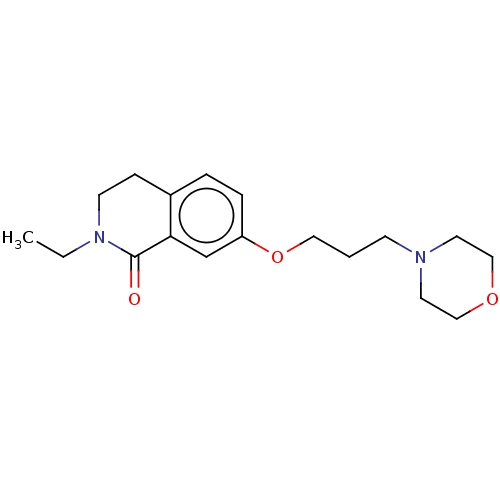 (CHEMBL4442619) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509932 (CHEMBL4526723) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 279 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509922 (CHEMBL4575319) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509930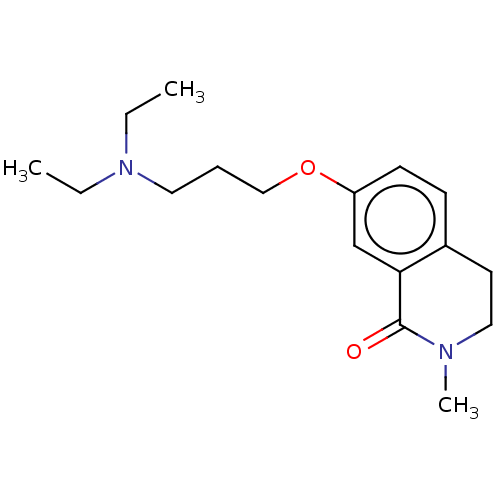 (CHEMBL4459304) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509931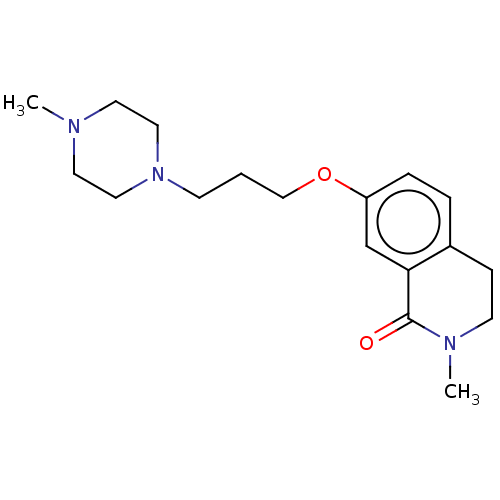 (CHEMBL4583068) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 706 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509927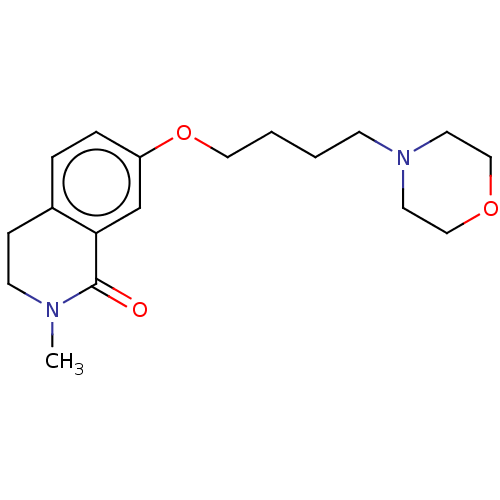 (CHEMBL4563108) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 856 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509928 (CHEMBL4583183) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 908 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50509934 (CHEMBL4557586) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from histamine H3 receptor in rat cerebral cortex homogenates incubated for 60 mins by Cheng and Prusoff... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509937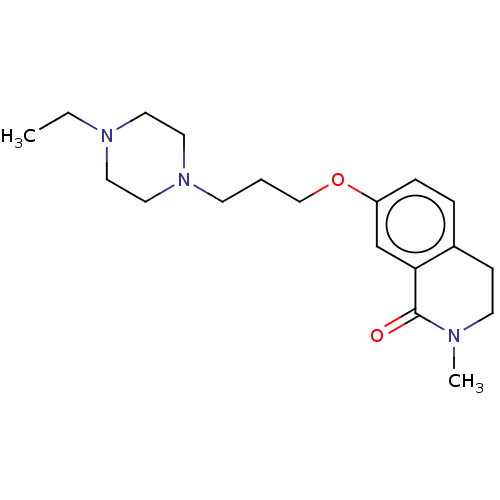 (CHEMBL4525456) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509935 (CHEMBL4576048) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50509926 (CHEMBL4437732) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangsu Marine Resources Development Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-mepyramine from histamine H1 receptor in guinea pig cerebellum homogenates incubated for 60 mins by Cheng and Prusoff equation a... | Bioorg Med Chem Lett 29: 1492-1496 (2019) Article DOI: 10.1016/j.bmcl.2019.04.015 BindingDB Entry DOI: 10.7270/Q21R6TTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535131 (WO2022013684, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | 6.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535140 (WO2022013684, Example 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM535170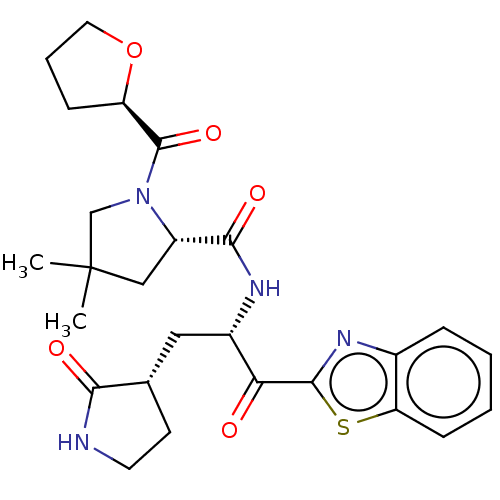 (WO2022013684, Example 20) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022013684 | >1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WD43SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM294911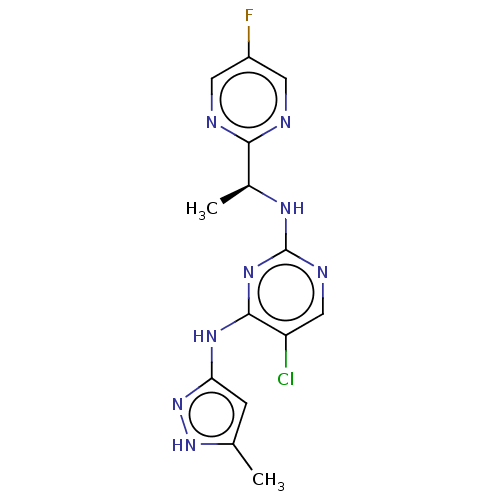 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50021656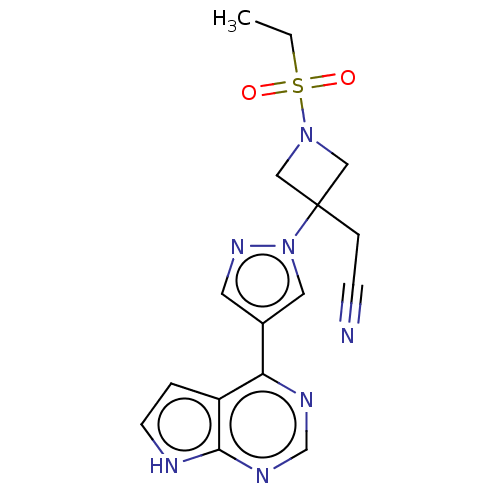 (BARICITINIB | INCB-028050 | LY-3009104 | US1011290...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM294911 (US10112907, Example 00020 | US10766894, Compound T...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50021656 (BARICITINIB | INCB-028050 | LY-3009104 | US1011290...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 273 total ) | Next | Last >> |