

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM22111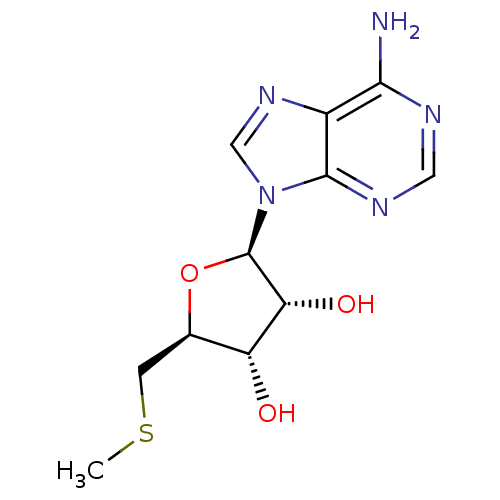 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from adenosine A1 receptor of rat cortical membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]R-PIA from adenosine A1 receptor of rat cortical membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-11 from human adenosine A3 receptor expressed in CHO cells | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]-MSX-2 from Adenosine A2A receptor of rat striatal membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]NECA from Adenosine A2A receptor of rat striatal membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50144902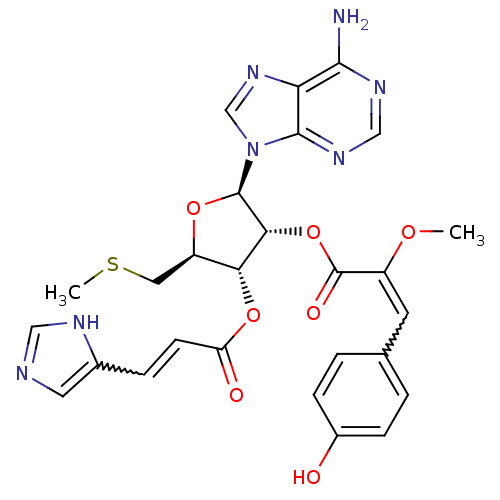 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from Adenosine A1 receptor of rat cortical membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-11 from human adenosine A3 receptor expressed in CHO cells | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Binding affinity towards human adenosine A2B receptor in VA13 fibroblasts as inhibition of adenylate cyclase at 10 uM; Less than 10% inhibition | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from rat Adenosine A1 receptor of cortical membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-298 from human adenosine A2B receptor expressed in HEK293 cells at 10 uM | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-298 from human adenosine A2B receptor expressed in HEK293 cells at 10 uM; Less than 10% inhibition | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]-MSX-2 from Adenosine A2A receptor of rat striatal membranes | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50094569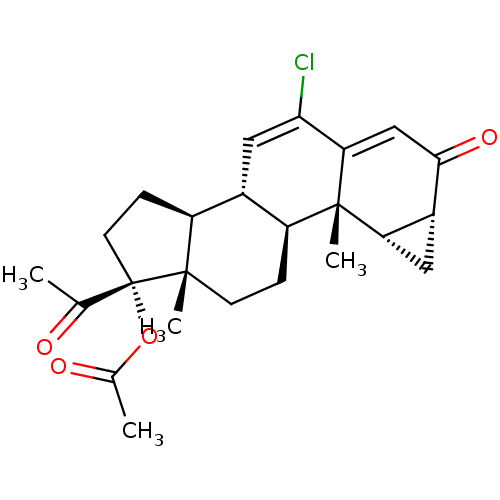 (6-chloro-3,20-dioxo-1beta,2beta-dihydro-3'H-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes without GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50094569 (6-chloro-3,20-dioxo-1beta,2beta-dihydro-3'H-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes with GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes without GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM31768 (CHEMBL295698 | Ketoconazole | Nizoral | Panfungol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50355307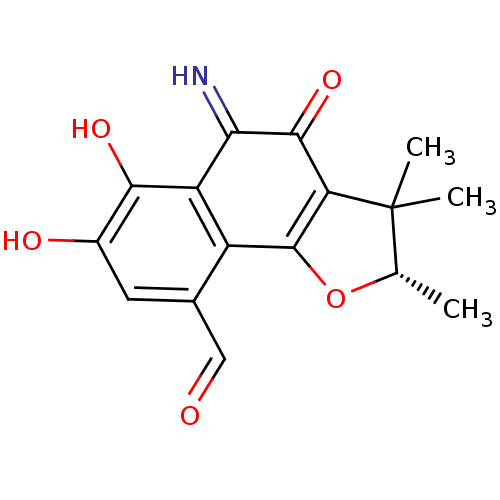 (CHEMBL1835012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase using MeOSuc-Ala-Ala-Pro-Val-para-nitroanilide chromogenic substrate after 10 mins spectrophotometrically | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of forskolin-stimulated cAMP accumulation in chinese hamster ovary cell membranes expressing human adenosine A3 receptor | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430553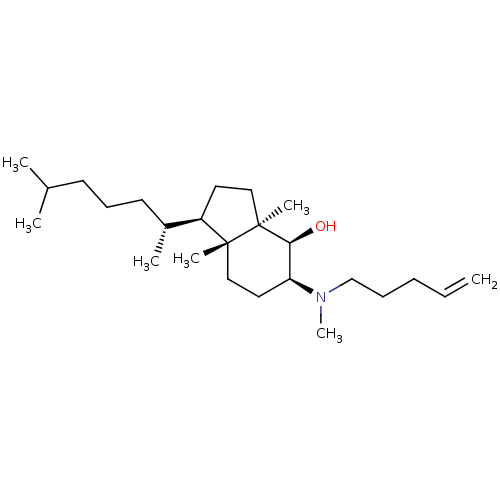 (CHEMBL2336919) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430556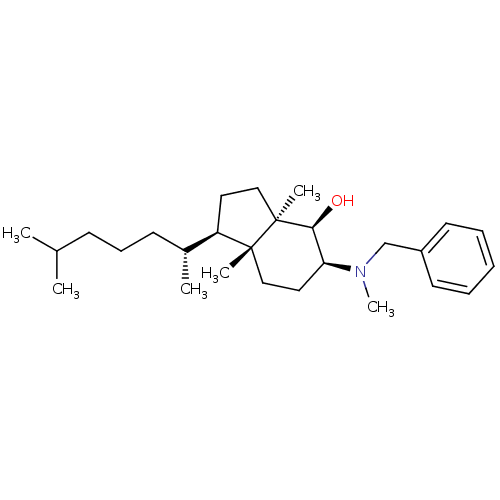 (CHEMBL2336934) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50192335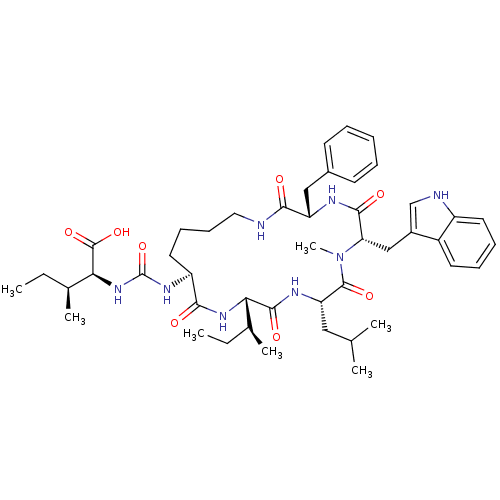 ((2S,3S)-2-{3-[(3S,6S,9S,12S,15S)-3-benzyl-12-((S)-...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis ptpb | J Med Chem 49: 4871-8 (2006) Article DOI: 10.1021/jm060327w BindingDB Entry DOI: 10.7270/Q2708124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50192337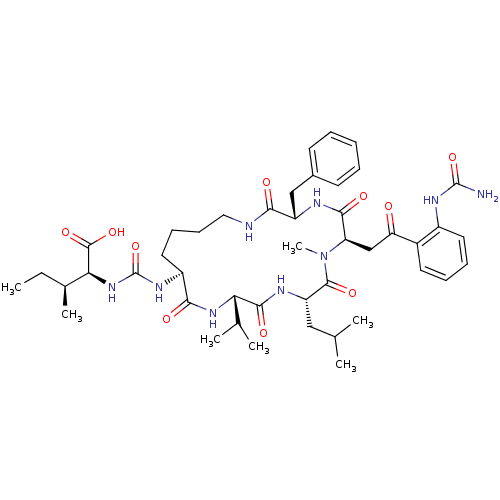 ((2S,3S)-2-(3-{(3S,6R,9S,12S,15S)-3-benzyl-9-isobut...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis ptpb | J Med Chem 49: 4871-8 (2006) Article DOI: 10.1021/jm060327w BindingDB Entry DOI: 10.7270/Q2708124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430558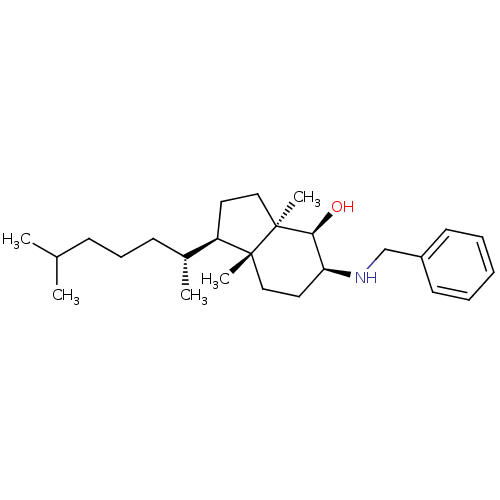 (CHEMBL2336925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50355305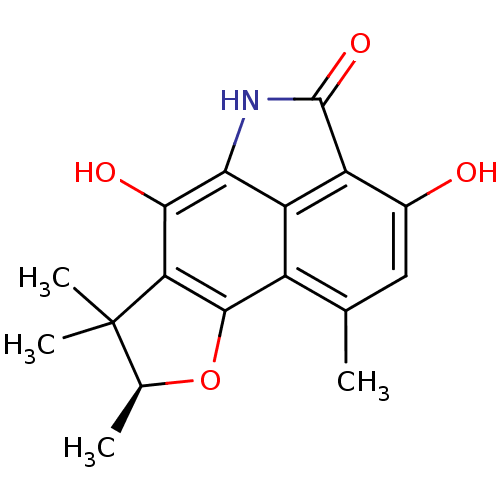 (CHEMBL1835010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase using MeOSuc-Ala-Ala-Pro-Val-para-nitroanilide chromogenic substrate after 10 mins spectrophotometrically | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430559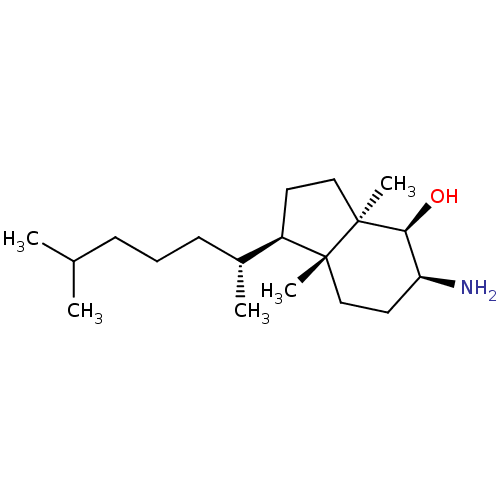 (CHEMBL2337344) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50009685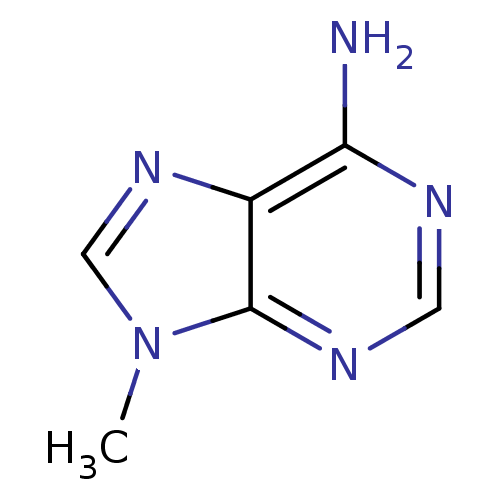 (9-METHYL-9H-PURIN-6-AMINE | 9-Methyl-9H-adenine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes without GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50009685 (9-METHYL-9H-PURIN-6-AMINE | 9-Methyl-9H-adenine | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes with GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430557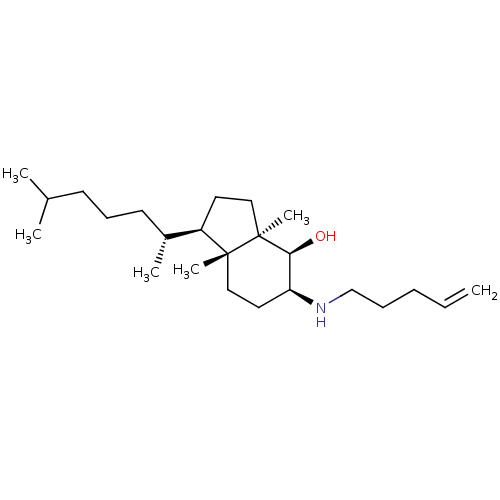 (CHEMBL2336928) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM22111 ((2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes with GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430554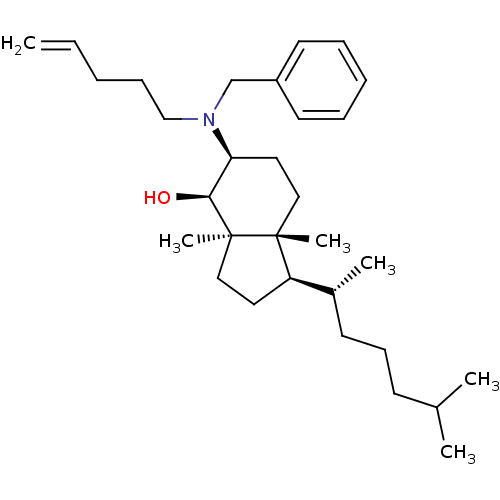 (CHEMBL2336918) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430560 (CHEMBL2336940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50355306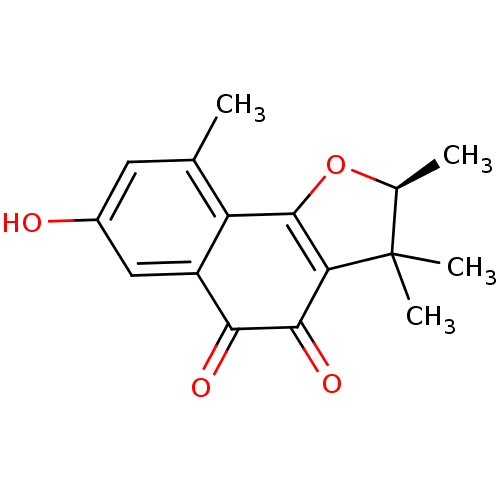 (CHEMBL1835011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase using MeOSuc-Ala-Ala-Pro-Val-para-nitroanilide chromogenic substrate after 10 mins spectrophotometrically | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50144902 (3-(4-Hydroxy-phenyl)-2-methoxy-acrylic acid (3S,5R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from adenosine A1 receptor of rat cortical membranes without GTP | J Med Chem 47: 2243-55 (2004) Article DOI: 10.1021/jm031092g BindingDB Entry DOI: 10.7270/Q2VQ33FT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50430555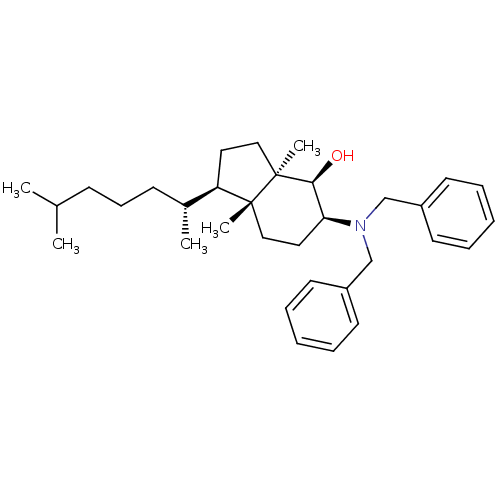 (CHEMBL2336917) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University Curated by ChEMBL | Assay Description Inhibition of delta8,7-sterol isomerase-mediated cholesterol biosynthesis in human HL60 cells assessed as increase in zymostenol accumulation | Bioorg Med Chem 21: 1925-43 (2013) Article DOI: 10.1016/j.bmc.2013.01.041 BindingDB Entry DOI: 10.7270/Q2SJ1N0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50204912 ((1S,3S,4S,4aS,9aS)-1,4,8-trihydroxy-6-methoxy-3,4a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human recombinant aromatase | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50355306 (CHEMBL1835011) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone oxidoreductase (Mus musculus) | BDBM50204915 ((1S,3S,4S,4aS,9aS)-1,4,8-trihydroxy-3,4a-dimethyl-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Induction of NAD(P)H:quinone reductase in mouse Hepa 1c1c7 cells | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50355307 (CHEMBL1835012) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human thrombin | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50355306 (CHEMBL1835011) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human thrombin | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50355305 (CHEMBL1835010) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human thrombin | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50355307 (CHEMBL1835012) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50355306 (CHEMBL1835011) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50355307 (CHEMBL1835012) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50355305 (CHEMBL1835010) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50355305 (CHEMBL1835010) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Carica papaya papain | J Nat Prod 74: 2282-5 (2011) Article DOI: 10.1021/np2004227 BindingDB Entry DOI: 10.7270/Q2ZW1M94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone oxidoreductase (Mus musculus) | BDBM50204913 (2-(2,6-dihydroxybenzoyl)-3-hydroxy-5-methylbenzoic...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Induction of NAD(P)H:quinone reductase in mouse Hepa 1c1c7 cells | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone oxidoreductase (Mus musculus) | BDBM50204916 (8-hydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylic ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Induction of NAD(P)H:quinone reductase in mouse Hepa 1c1c7 cells | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone oxidoreductase (Mus musculus) | BDBM50204912 ((1S,3S,4S,4aS,9aS)-1,4,8-trihydroxy-6-methoxy-3,4a...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Induction of NAD(P)H:quinone reductase in mouse Hepa 1c1c7 cells | J Nat Prod 70: 353-60 (2007) Article DOI: 10.1021/np060505o BindingDB Entry DOI: 10.7270/Q2930STQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50192336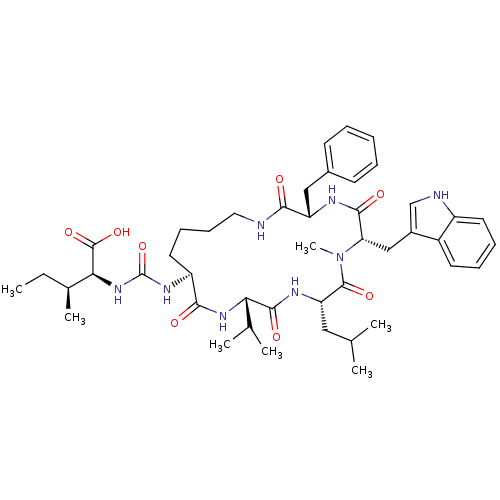 ((2S,3S)-2-{3-[(3S,6S,9S,12S,15S)-3-benzyl-6-(1H-in...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis ptpb | J Med Chem 49: 4871-8 (2006) Article DOI: 10.1021/jm060327w BindingDB Entry DOI: 10.7270/Q2708124 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 69 total ) | Next | Last >> |