

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin D (Homo sapiens (Human)) | BDBM50393892 (CHEMBL2158253) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393886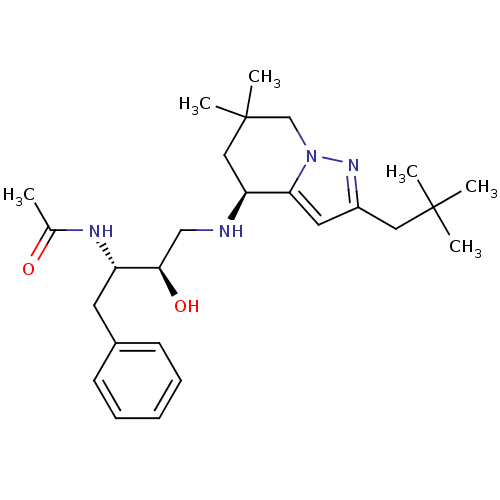 (CHEMBL2158259) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393888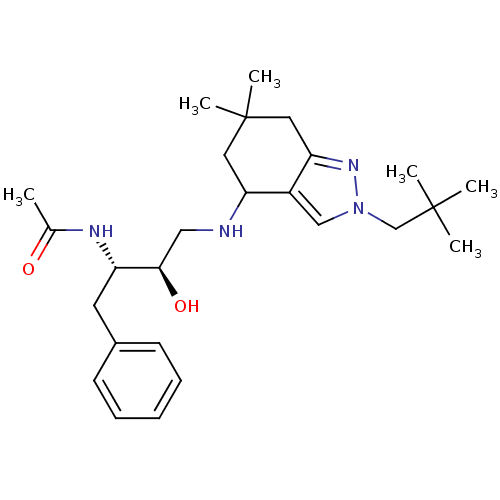 (CHEMBL2158256) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393889 (CHEMBL2158252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393887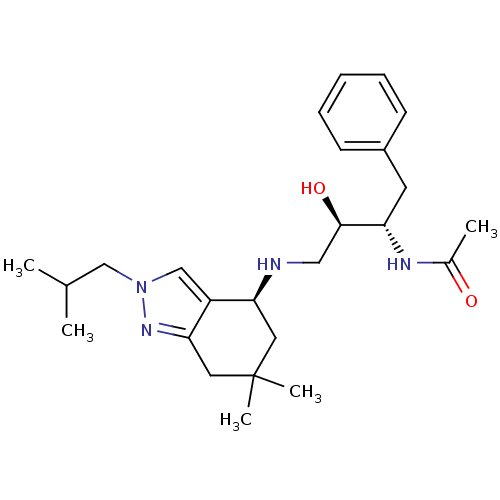 (CHEMBL2158257) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393890 (CHEMBL2158255) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393891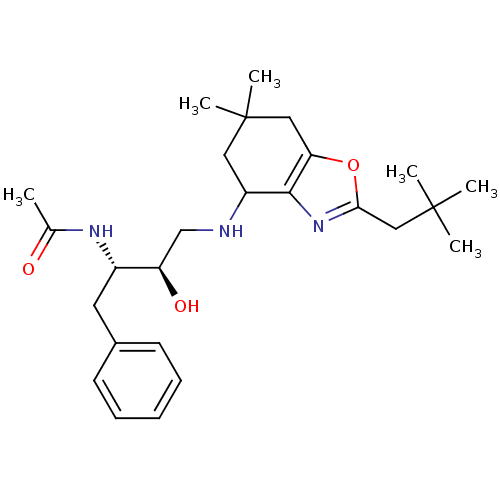 (CHEMBL2158254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50393893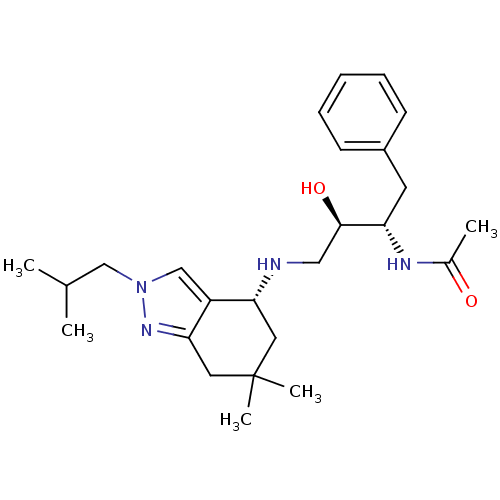 (CHEMBL2158258) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human cathepsin D using DAB-CYL-Glu-ArG-Nle-Phe-Leu-Ser-Phe-Pro-EDANS incubated for 20 mins prior to substrate addition measured after ... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393892 (CHEMBL2158253) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393889 (CHEMBL2158252) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393892 (CHEMBL2158253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393889 (CHEMBL2158252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393889 (CHEMBL2158252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393892 (CHEMBL2158253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393887 (CHEMBL2158257) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393887 (CHEMBL2158257) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393888 (CHEMBL2158256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393888 (CHEMBL2158256) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393888 (CHEMBL2158256) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393886 (CHEMBL2158259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393887 (CHEMBL2158257) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393890 (CHEMBL2158255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393885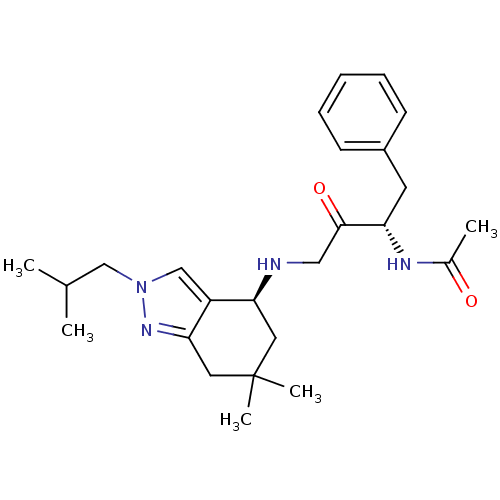 (CHEMBL2158260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393890 (CHEMBL2158255) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393886 (CHEMBL2158259) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393893 (CHEMBL2158258) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393890 (CHEMBL2158255) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393893 (CHEMBL2158258) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50393891 (CHEMBL2158254) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393891 (CHEMBL2158254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393891 (CHEMBL2158254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of human BACE1 expressed in Escherichia coli BL21(DE3) using Eu-EVNLDAEFK as substrate incubated for 30 mins prior to substrate addition m... | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50393885 (CHEMBL2158260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Medivir AB Curated by ChEMBL | Assay Description Inhibition of BACE1 in human HEK cells expressing APP Swedish mutant assessed as reduction in amyloid beta40 level by ELISA | Bioorg Med Chem Lett 22: 6721-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.097 BindingDB Entry DOI: 10.7270/Q2MS3TVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294056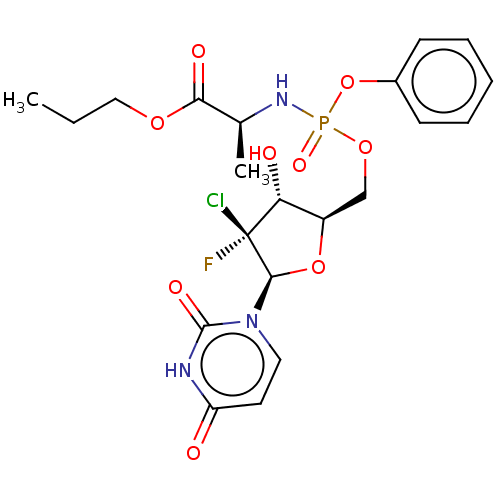 (US10106571, Example 14) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 76 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294057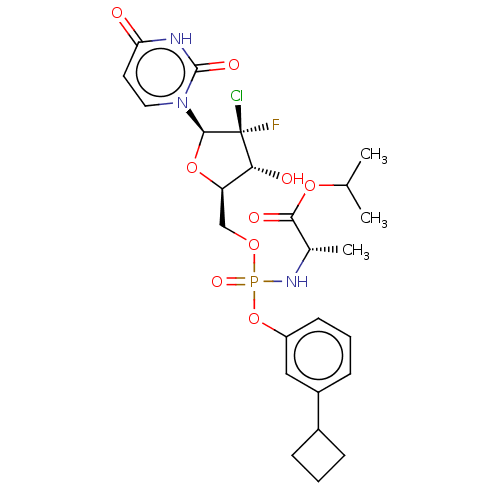 (US10106571, Example 15) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294058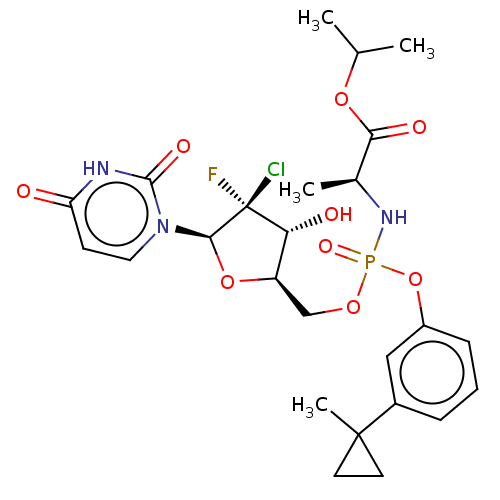 (US10106571, Example 16) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 91 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294059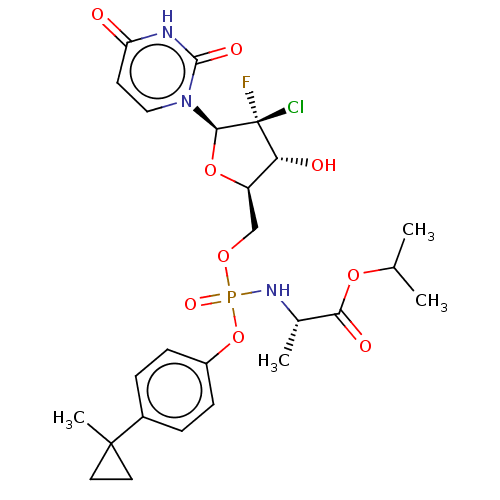 (US10106571, Example 17) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294060 (US10106571, Example 20) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 65 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294061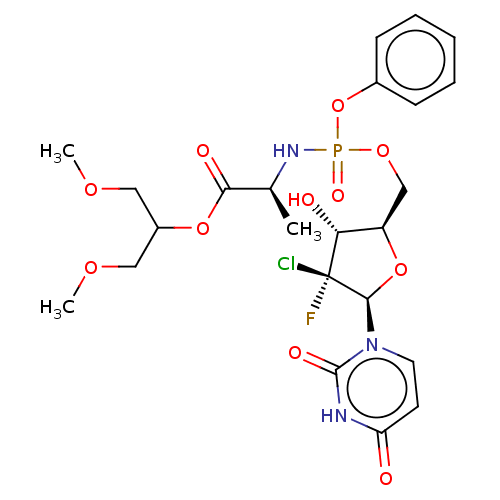 (US10106571, Example 21) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294062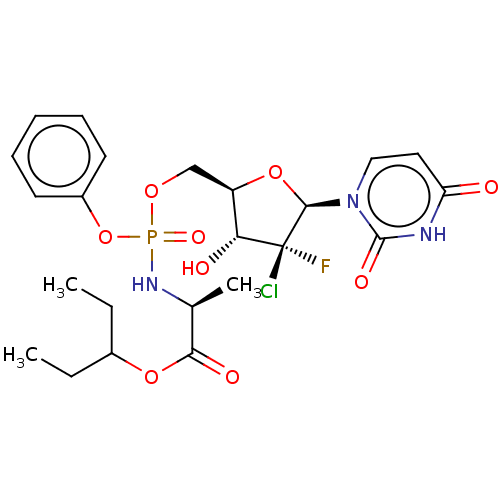 (US10106571, Example 22) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294045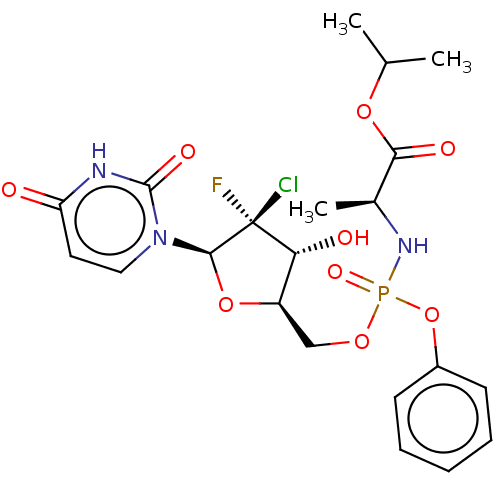 (US10106571, Example 2 | US10106571, Example 23) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294064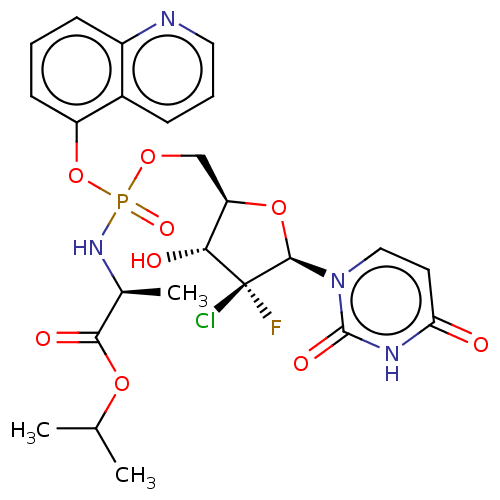 (US10106571, Example 24) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294065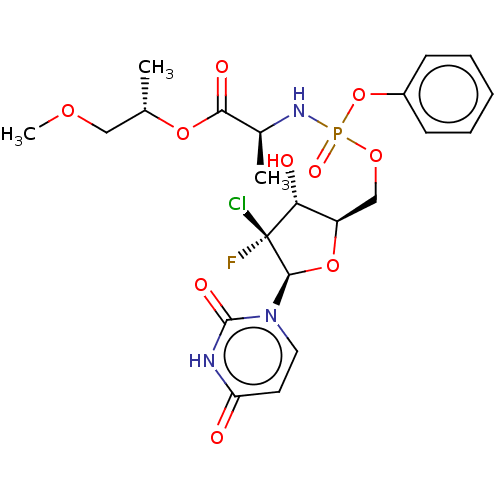 (US10106571, Example 25) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294055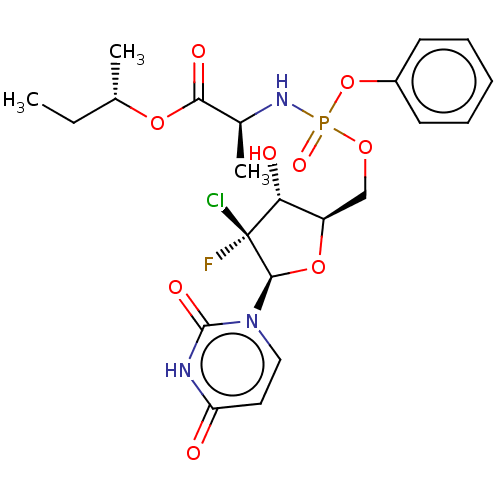 (US10106571, Example 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 61 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294054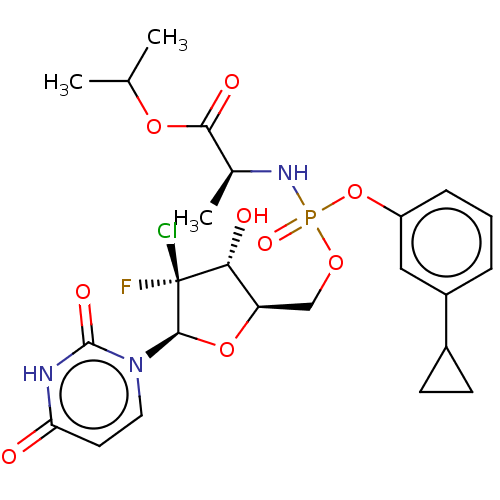 (US10106571, Example 12) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294053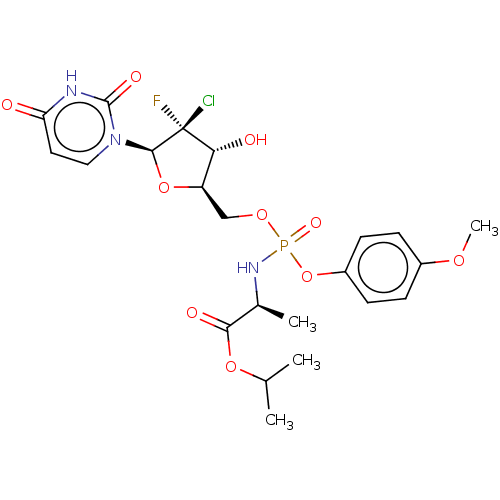 (US10106571, Example 11) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294052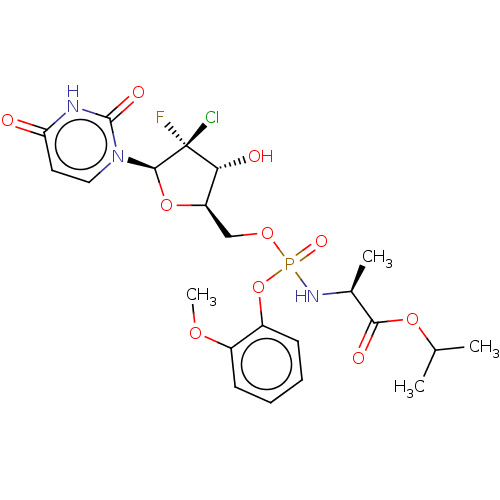 (US10106571, Example 10) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294051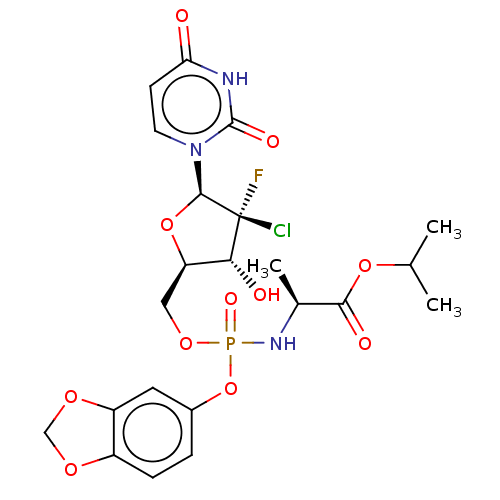 (US10106571, Example 9) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294050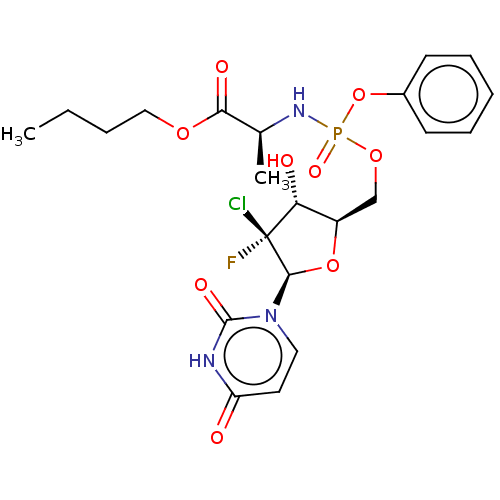 (US10106571, Example 8) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294048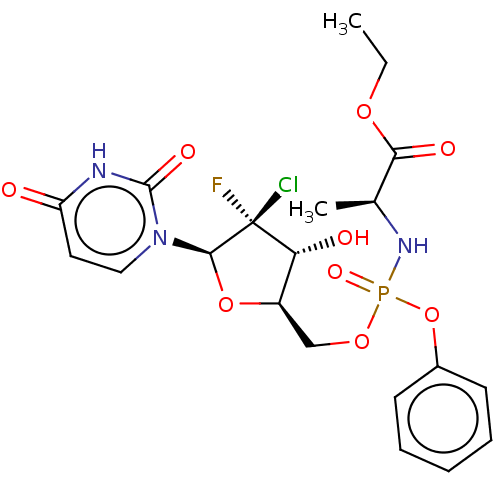 (US10106571, Example 6) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM294047 (US10106571, Example 5) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
MEDIVIR AB US Patent | Assay Description The assay utilizes the stably transfected cell line Huh-7 luc/neo (hereafter referred to as Huh-Luc). This cell line harbors an RNA encoding a bicist... | US Patent US10106571 (2018) BindingDB Entry DOI: 10.7270/Q2NP26GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |