

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein cereblon (Homo sapiens (Human)) | BDBM50541818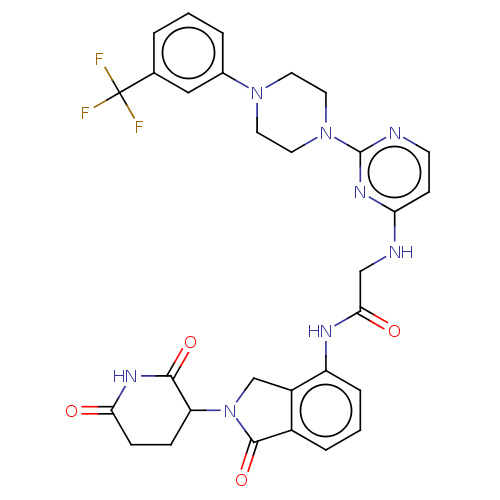 (CHEMBL4648907 | US11530219, Compound 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon (Homo sapiens (Human)) | BDBM50541817 (CHEMBL4635421 | US11530219, Compound 14) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon (Homo sapiens (Human)) | BDBM50541820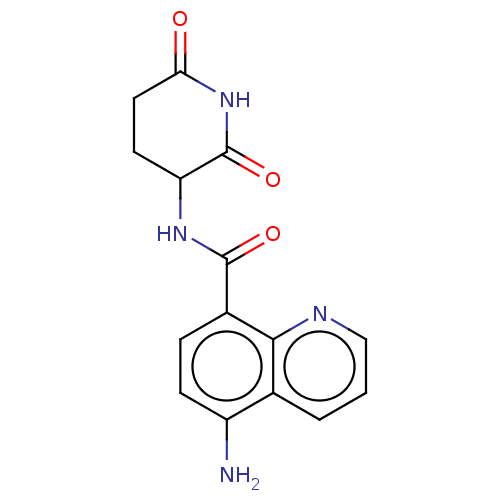 (CHEMBL4648134) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon (Homo sapiens (Human)) | BDBM50541819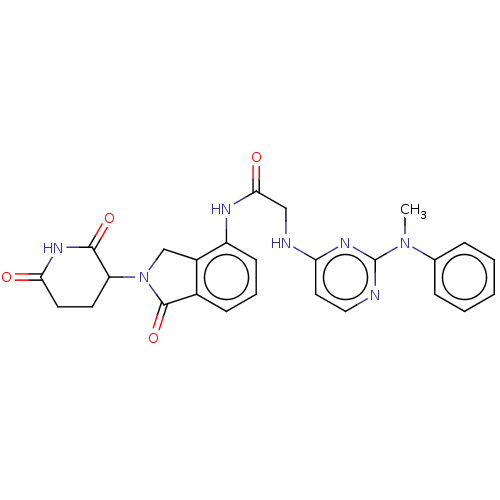 (CHEMBL4645406 | US11530219, Compound 17) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549967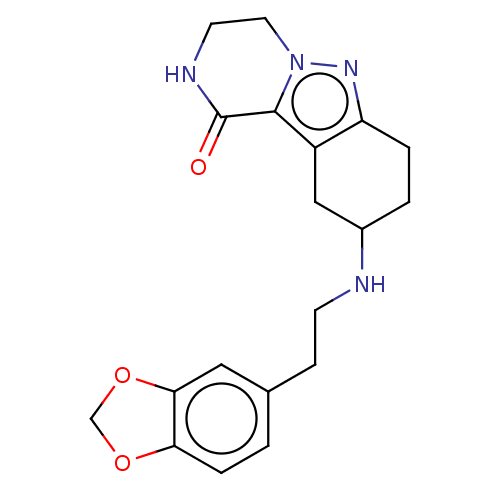 (CHEMBL4800330) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549969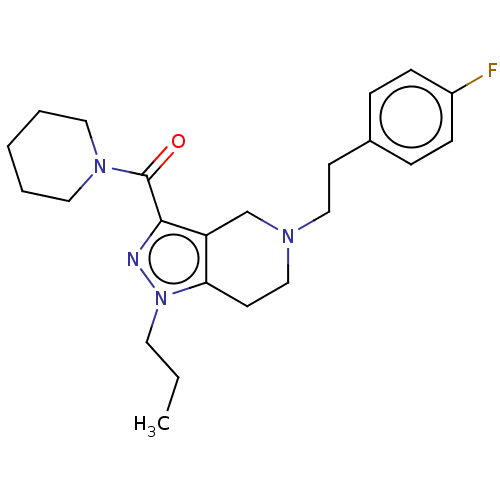 (CHEMBL4795855) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549961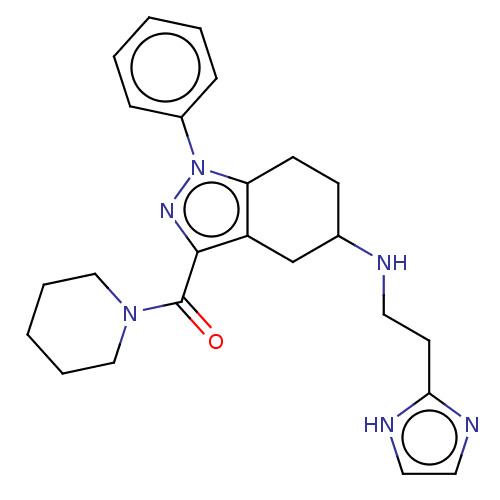 (CHEMBL4749047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549962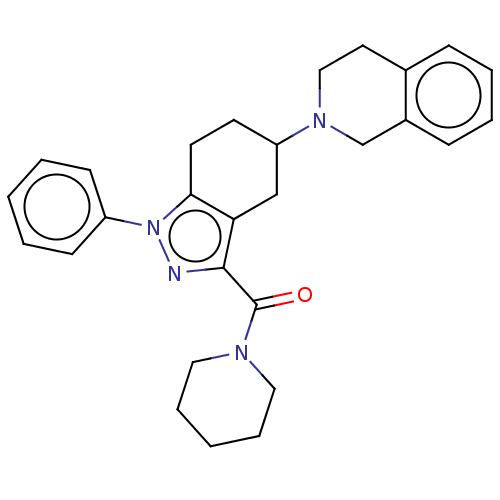 (CHEMBL4764493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549963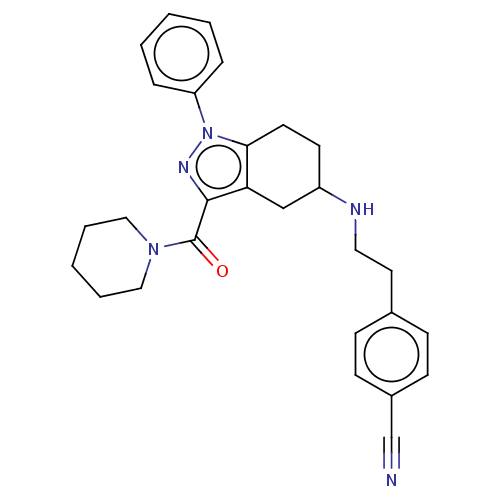 (CHEMBL4754389) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549964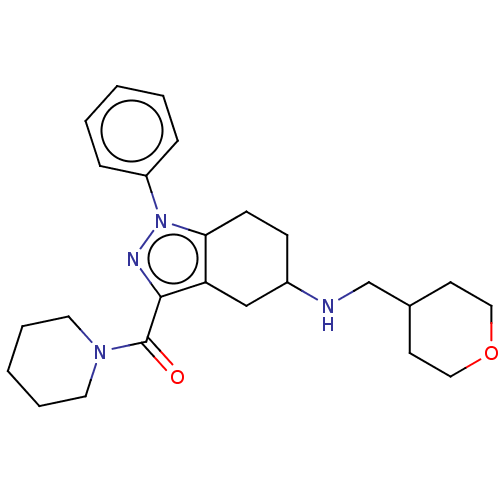 (CHEMBL4793307) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549965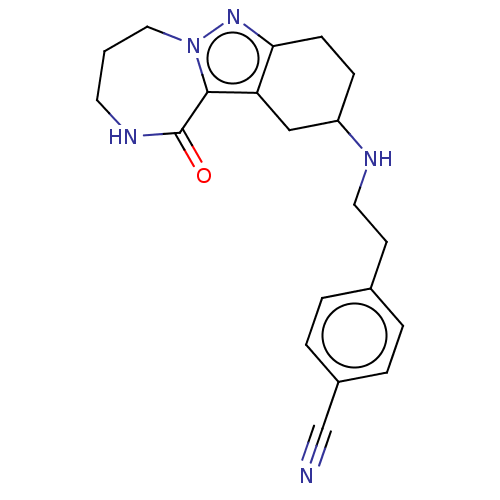 (CHEMBL4741458) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549966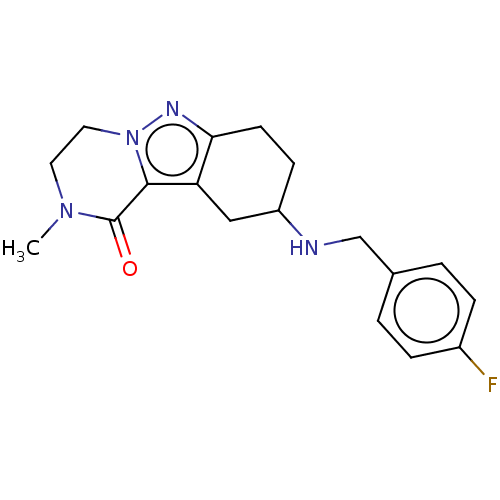 (CHEMBL4745019) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549970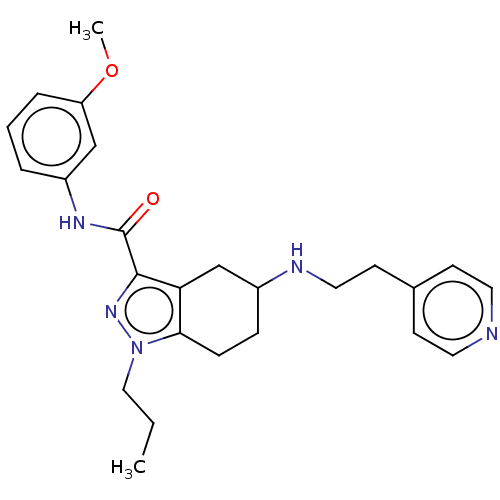 (CHEMBL4752119) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549968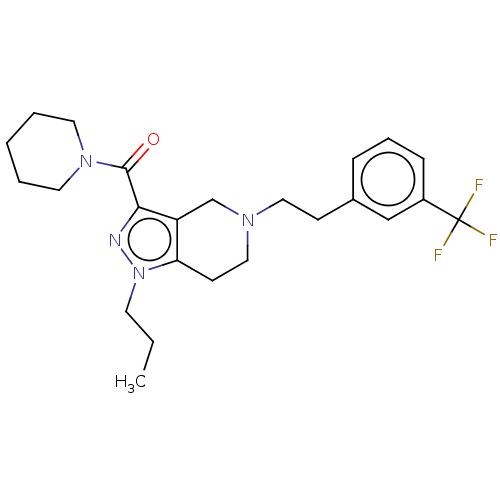 (CHEMBL4781851) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549969 (CHEMBL4795855) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50549970 (CHEMBL4752119) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 1 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549961 (CHEMBL4749047) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549962 (CHEMBL4764493) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549963 (CHEMBL4754389) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549964 (CHEMBL4793307) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549965 (CHEMBL4741458) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549966 (CHEMBL4745019) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549967 (CHEMBL4800330) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Homo sapiens (Human)) | BDBM50549968 (CHEMBL4781851) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to sigma 2 receptor (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00002 BindingDB Entry DOI: 10.7270/Q2M61PV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein cereblon (Homo sapiens (Human)) | BDBM65454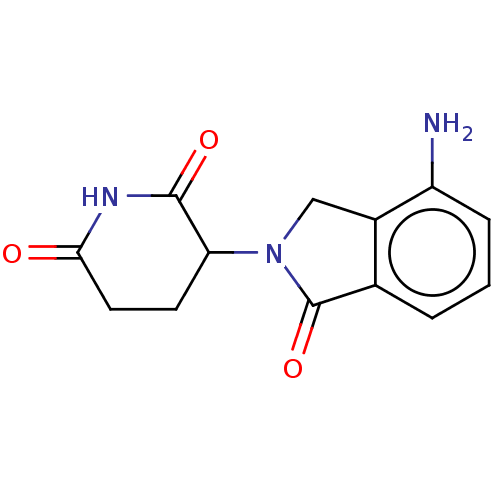 (191732-72-6 | CC-5013 | Lenalidomide | Revimid | R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein cereblon (Homo sapiens (Human)) | BDBM50541821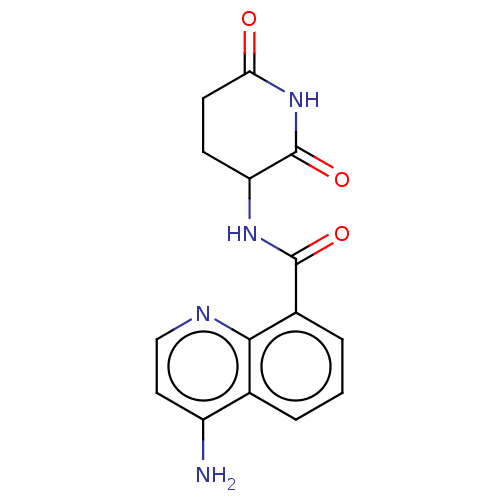 (CHEMBL4639892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Binding affinity to CBRN (unknown origin) incubated for 60 mins by lenalidomide displacement assay | ACS Med Chem Lett 11: 1088-1089 (2020) Article DOI: 10.1021/acsmedchemlett.0c00214 BindingDB Entry DOI: 10.7270/Q2VH5SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502491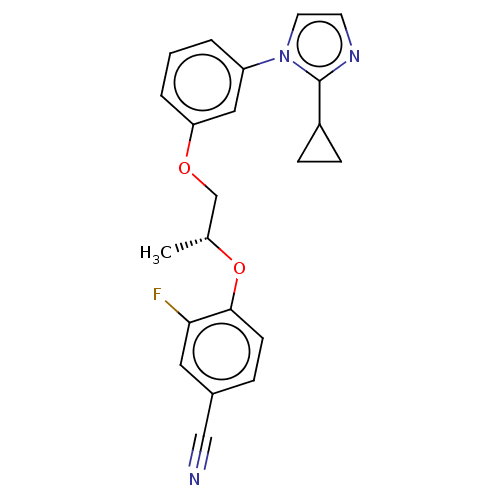 (CHEMBL4443354 | US11834430, Compound 41) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-coenzyme A synthetase, cytoplasmic (Homo sapiens) | BDBM50517061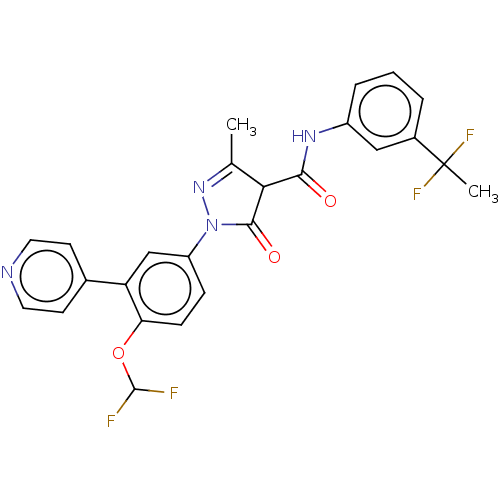 (CHEMBL4467131) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ACSS2 in human MDA-MB-468 cells assessed as 13C-acetate incorporation incubated for 5 hrs measured under hypoxic conditions by LCMS ana... | ACS Med Chem Lett 10: 1100-1101 (2019) Article DOI: 10.1021/acsmedchemlett.9b00295 BindingDB Entry DOI: 10.7270/Q2J969Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502493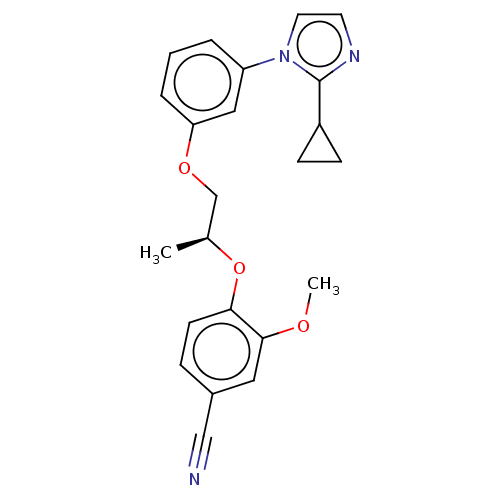 (CHEMBL4558383 | US11834430, Compound 81) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502489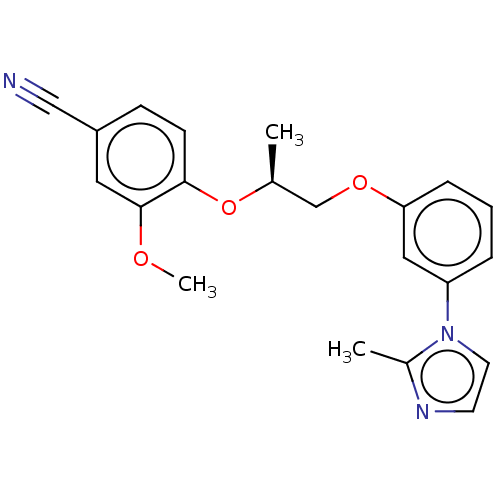 (CHEMBL4583704 | US11834430, Compound 72) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579603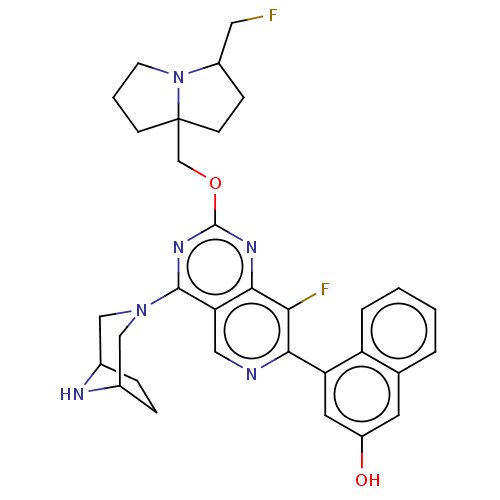 (CHEMBL4855757 | US11453683, Example 384 | US202302...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502494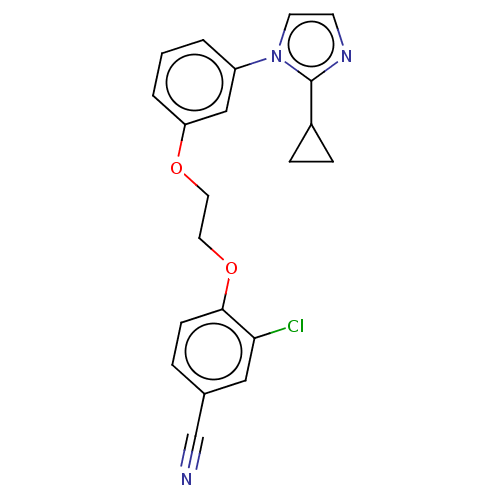 (CHEMBL4446351 | US11834430, Compound 9) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50587586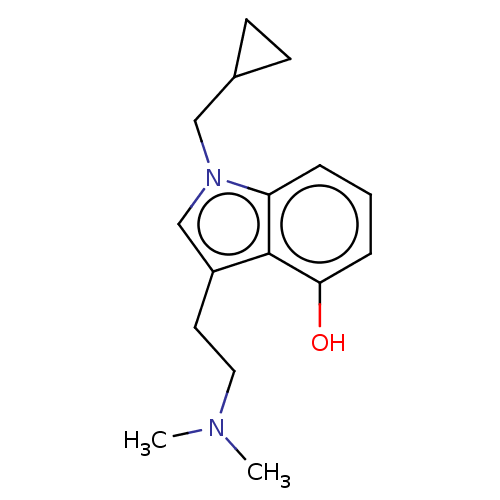 (CHEMBL5078731) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of human 5HT2C expressed in HEK293T cells co-transfected with Galphaq-RLuc8, Ggamma1-GFP2 and Gbeta1 assessed as dissociation of Galphaq f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00578 BindingDB Entry DOI: 10.7270/Q2BR8X3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502488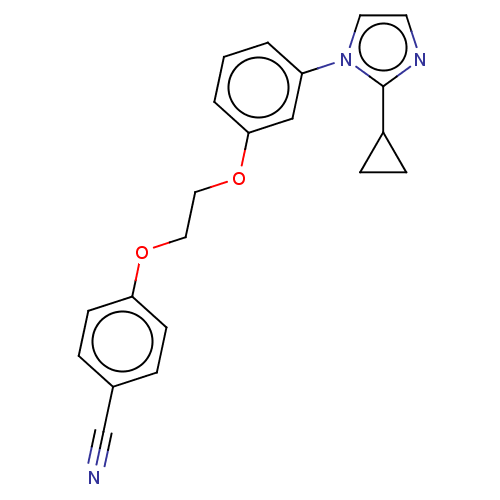 (CHEMBL4449942 | US11834430, Compound 62) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502492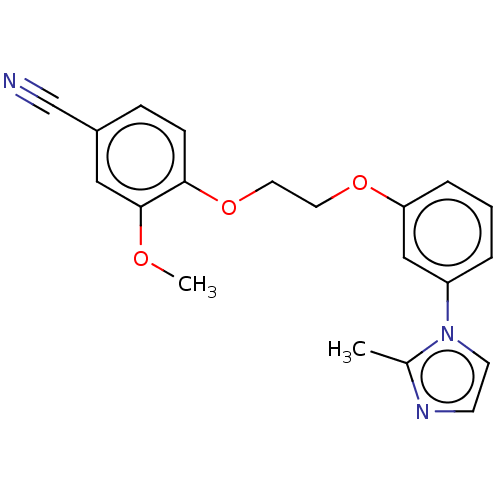 (CHEMBL4446048 | US11834430, Compound 8) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-S-isoprenylcysteine O-methyltransferase (Homo sapiens (Human)) | BDBM50502486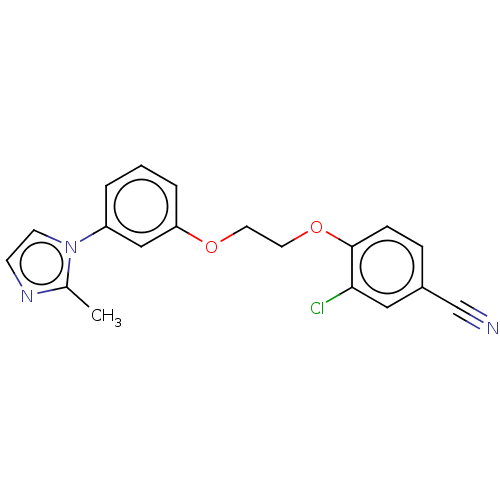 (CHEMBL4468997 | US11834430, Compound 5) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human ICMT expressed in baculovirus infected Sf9 insect cells using SAM as substrate preincubated for 30 mins followed by s... | ACS Med Chem Lett 10: 1024-1025 (2019) Article DOI: 10.1021/acsmedchemlett.9b00269 BindingDB Entry DOI: 10.7270/Q2FN19F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579600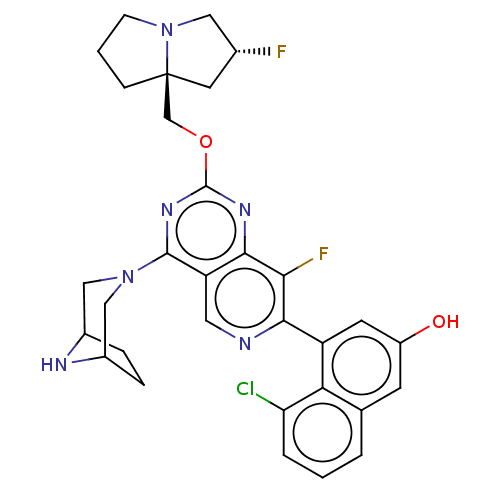 (CHEMBL4857438 | US11453683, Example 251 | US202302...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50587589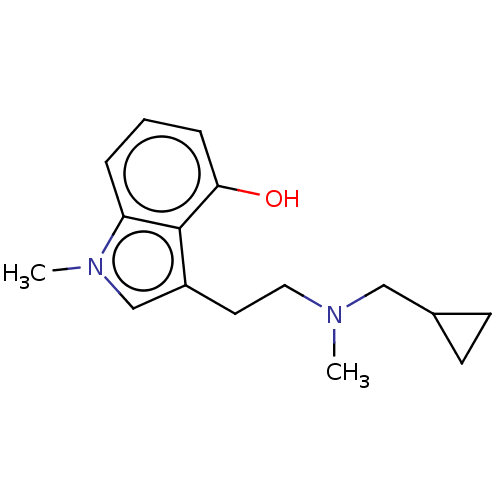 (CHEMBL5081637) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.245 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Modulation of human 5HT2B expressed in HEK293T cells co-transfected with Galphaq-RLuc8, Ggamma1-GFP2 and Gbeta1 assessed as dissociation of Galphaq f... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00578 BindingDB Entry DOI: 10.7270/Q2BR8X3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50520569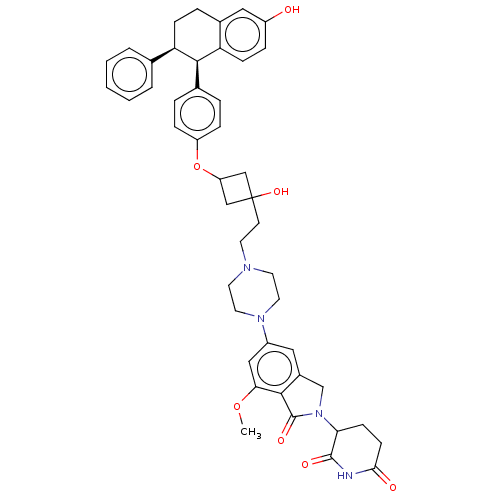 (CHEMBL4456119) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ERalpha in human T47D cells by ERE-driven luciferase reporter gene assay based target engagement assay | ACS Med Chem Lett 11: 407-408 (2020) Article DOI: 10.1021/acsmedchemlett.0c00071 BindingDB Entry DOI: 10.7270/Q28S4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579595 (CHEMBL4863339 | US11453683, Example 185 | US202302...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-coenzyme A synthetase, cytoplasmic (Homo sapiens) | BDBM50517065 (CHEMBL4550594) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ACSS2 in human MDA-MB-468 cells assessed as 13C-acetate incorporation incubated for 5 hrs measured under hypoxic conditions by LCMS ana... | ACS Med Chem Lett 10: 1100-1101 (2019) Article DOI: 10.1021/acsmedchemlett.9b00295 BindingDB Entry DOI: 10.7270/Q2J969Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50520563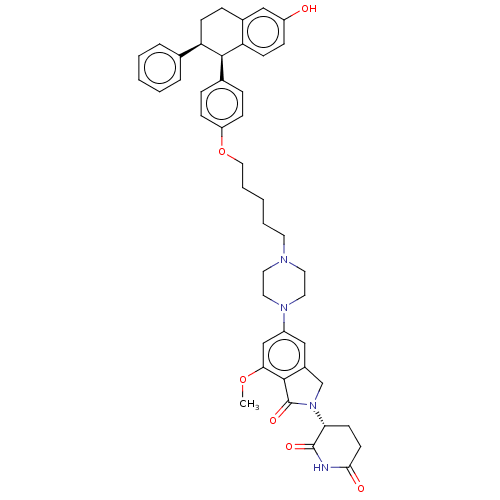 (CHEMBL4537710) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ERalpha in human T47D cells by ERE-driven luciferase reporter gene assay based target engagement assay | ACS Med Chem Lett 11: 407-408 (2020) Article DOI: 10.1021/acsmedchemlett.0c00071 BindingDB Entry DOI: 10.7270/Q28S4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM148993 (US8962648, 72 | US8962648, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579597 (CHEMBL4876040 | US11453683, Example 243 | US202302...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579601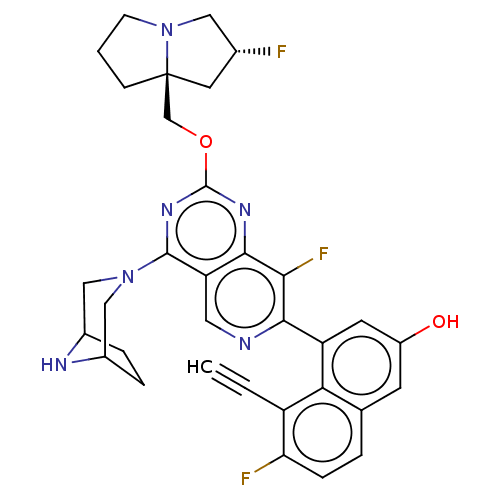 (CHEMBL4858364 | US11453683, Example 252 | US202302...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50520581 (CHEMBL4208017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Induction of ERalpha protein degradation in human MCF7 cells assessed as reduction in ERalpha protein levels incubated for 24 hrs by In-cell western ... | ACS Med Chem Lett 11: 412-413 (2020) Article DOI: 10.1021/acsmedchemlett.0c00107 BindingDB Entry DOI: 10.7270/Q21839W6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50579594 (CHEMBL4859236 | US11453683, Example 36 | US2023027...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Cy5-labelled tracer from biotinylated GDP-loaded human recombinant KRAS G12D mutant (1 to 169 residues) measured after 60 mins by TR-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00545 BindingDB Entry DOI: 10.7270/Q2V69PDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lymphokine-activated killer T-cell-originated protein kinase (Homo sapiens (Human)) | BDBM149054 (US8962648, 319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | 7.4 | 25 |
OncoTherapy Science, Inc. US Patent | Assay Description PBK activity was determined in the presence or absence of compounds using fluorescein isothiocyanate-labeled (FITC-labeled) histone H3 peptide as a s... | US Patent US8962648 (2015) BindingDB Entry DOI: 10.7270/Q27H1H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-coenzyme A synthetase, cytoplasmic (Homo sapiens) | BDBM50517069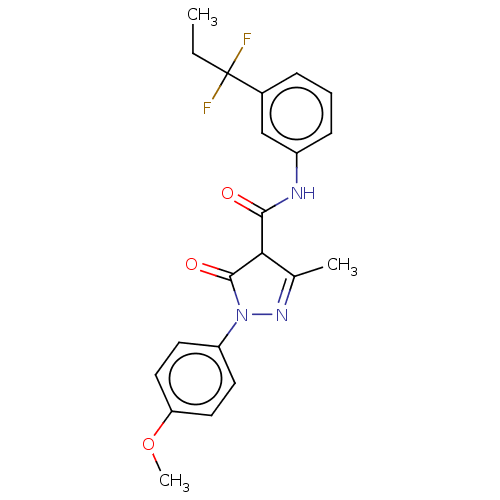 (CHEMBL4449652) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.463 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ACSS2 in human MDA-MB-468 cells assessed as 13C-acetate incorporation incubated for 5 hrs measured under hypoxic conditions by LCMS ana... | ACS Med Chem Lett 10: 1100-1101 (2019) Article DOI: 10.1021/acsmedchemlett.9b00295 BindingDB Entry DOI: 10.7270/Q2J969Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50520566 (CHEMBL4458123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Usona Institute Curated by ChEMBL | Assay Description Inhibition of ERalpha in human T47D cells by ERE-driven luciferase reporter gene assay based target engagement assay | ACS Med Chem Lett 11: 407-408 (2020) Article DOI: 10.1021/acsmedchemlett.0c00071 BindingDB Entry DOI: 10.7270/Q28S4T9H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1059 total ) | Next | Last >> |