 Found 359 hits with Last Name = 'kaur' and Initial = 'm'
Found 359 hits with Last Name = 'kaur' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50515758

(CHEMBL4453575)Show SMILES O=C(OC[C@@H]1CO1)c1cccc2c1[nH]c1ccccc1c2=O |r| Show InChI InChI=1S/C17H13NO4/c19-16-11-4-1-2-7-14(11)18-15-12(16)5-3-6-13(15)17(20)22-9-10-8-21-10/h1-7,10H,8-9H2,(H,18,19)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase assessed as inhibition constant by UV-Vis spectra based Benesi Hilebrand equation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126631
BindingDB Entry DOI: 10.7270/Q2RB780V |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50050769
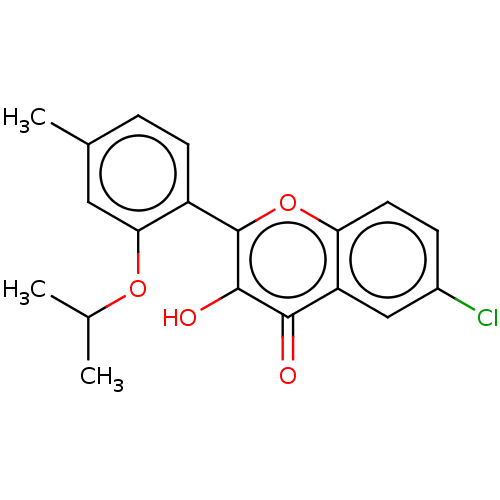
(CHEMBL3318019)Show InChI InChI=1S/C19H17ClO4/c1-10(2)23-16-8-11(3)4-6-13(16)19-18(22)17(21)14-9-12(20)5-7-15(14)24-19/h4-10,22H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A3 receptor |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Homo sapiens (Human)) | BDBM50515763
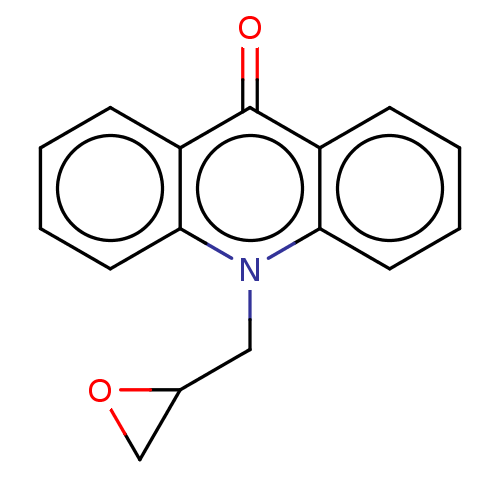
(CHEMBL4458303)Show InChI InChI=1S/C16H13NO2/c18-16-12-5-1-3-7-14(12)17(9-11-10-19-11)15-8-4-2-6-13(15)16/h1-8,11H,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human dihydrofolate reductase assessed as inhibition constant by UV-Vis spectra based Benesi Hilebrand equation analysis |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.126631
BindingDB Entry DOI: 10.7270/Q2RB780V |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Xanthine dehydrogenase/oxidase
(Homo sapiens (Human)) | BDBM50016784
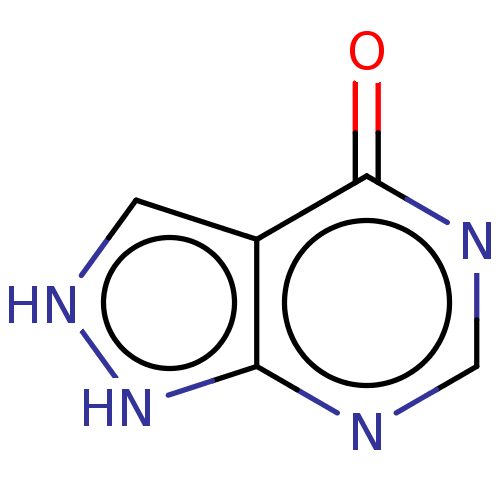
(ALLOPURINOL | BW-56-158 | BW-56158 | CHEBI:40279 |...)Show InChI InChI=1S/C5H2N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of xanthine oxidase (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Cystic fibrosis transmembrane conductance regulator
(Homo sapiens (Human)) | BDBM7458

(5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...)Show InChI InChI=1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of forskolin-stimulated CFTR (unknown origin) |
Eur J Med Chem 84: 206-39 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.013
BindingDB Entry DOI: 10.7270/Q2GH9KMN |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50613673

(CHEMBL1964259)Show SMILES OCCOCCNS(=O)(=O)c1ccc(N\C=C2/C(=O)Nc3ccc4ncsc4c23)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613597
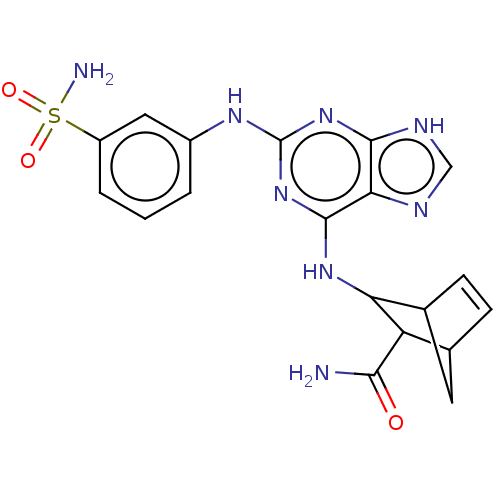
(CHEMBL5270626)Show SMILES NC(=O)C1C2CC(C=C2)C1Nc1nc(Nc2cccc(c2)S(N)(=O)=O)nc2[nH]cnc12 |c:7| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613596
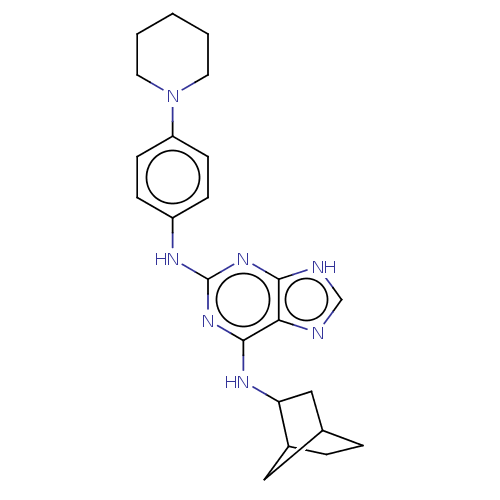
(CHEMBL5272356)Show SMILES C1CC2CC1CC2Nc1nc(Nc2ccc(cc2)N2CCCCC2)nc2[nH]cnc12 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613595

(CHEMBL5272100)Show SMILES CC(C)Oc1cccc(Nc2nc(NC3C4CC(C=C4)C3C(N)=O)c3nc[nH]c3n2)c1 |c:18| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613594
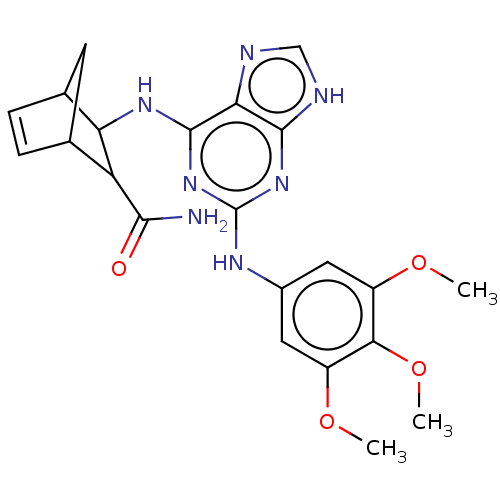
(CHEMBL5275755)Show SMILES COc1cc(Nc2nc(NC3C4CC(C=C4)C3C(N)=O)c3nc[nH]c3n2)cc(OC)c1OC |c:14| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613593
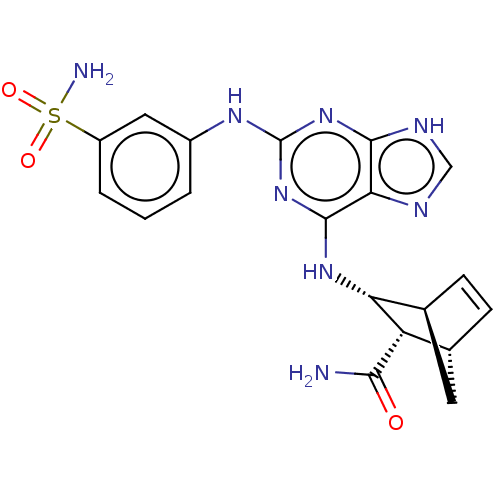
(CHEMBL5285324)Show SMILES [H][C@@]12C[C@@]([H])(C=C1)[C@@H]([C@@H]2Nc1nc(Nc2cccc(c2)S(N)(=O)=O)nc2[nH]cnc12)C(N)=O |r,c:5| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126727
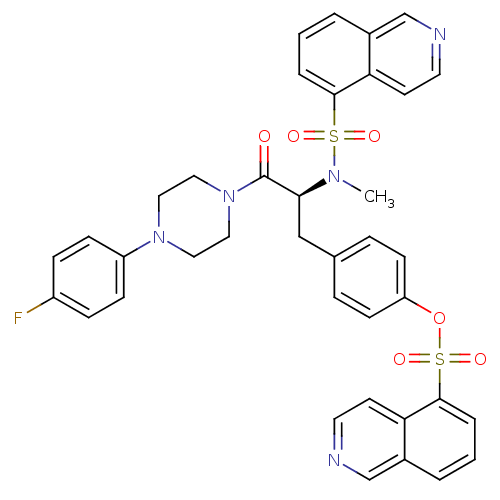
((S)-4-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-(N-m...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccc(F)cc1)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C38H34FN5O6S2/c1-42(51(46,47)36-6-2-4-28-25-40-18-16-33(28)36)35(38(45)44-22-20-43(21-23-44)31-12-10-30(39)11-13-31)24-27-8-14-32(15-9-27)50-52(48,49)37-7-3-5-29-26-41-19-17-34(29)37/h2-19,25-26,35H,20-24H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50044262

(CHEMBL3314084)Show SMILES Clc1cccc(Cl)c1N1C(=O)\C(=C\c2coc3ccccc3c2=O)c2ccccc12 Show InChI InChI=1S/C24H13Cl2NO3/c25-18-8-5-9-19(26)22(18)27-20-10-3-1-6-15(20)17(24(27)29)12-14-13-30-21-11-4-2-7-16(21)23(14)28/h1-13H/b17-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in prostaglandin production using arachidonic acid as substrate incubated with enzyme for ... |
Eur J Med Chem 77: 185-92 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.003
BindingDB Entry DOI: 10.7270/Q2319XH6 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397869

(CHEMBL2179502)Show SMILES CN(C)S(=O)(=O)CCCn1c2CCCCc2c2cc(ccc12)-c1nc(C)no1 Show InChI InChI=1S/C20H26N4O3S/c1-14-21-20(27-22-14)15-9-10-19-17(13-15)16-7-4-5-8-18(16)24(19)11-6-12-28(25,26)23(2)3/h9-10,13H,4-8,11-12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397870

(CHEMBL2179501)Show SMILES Cc1noc(n1)-c1ccc2n(CCCOc3nc(C)cc(n3)C(F)(F)F)c3CCCCc3c2c1 Show InChI InChI=1S/C24H24F3N5O2/c1-14-12-21(24(25,26)27)30-23(28-14)33-11-5-10-32-19-7-4-3-6-17(19)18-13-16(8-9-20(18)32)22-29-15(2)31-34-22/h8-9,12-13H,3-7,10-11H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397871

(CHEMBL2179500)Show SMILES CCc1cc(CC)nc(OCCCn2c3CCCCc3c3cc(ccc23)-c2nc(C)no2)n1 Show InChI InChI=1S/C26H31N5O2/c1-4-19-16-20(5-2)29-26(28-19)32-14-8-13-31-23-10-7-6-9-21(23)22-15-18(11-12-24(22)31)25-27-17(3)30-33-25/h11-12,15-16H,4-10,13-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397872

(CHEMBL2179499)Show SMILES Cc1noc(n1)-c1ccc2n(CCCOc3nc(C)cc(C)n3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H27N5O2/c1-15-13-16(2)26-24(25-15)30-12-6-11-29-21-8-5-4-7-19(21)20-14-18(9-10-22(20)29)23-27-17(3)28-31-23/h9-10,13-14H,4-8,11-12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397879
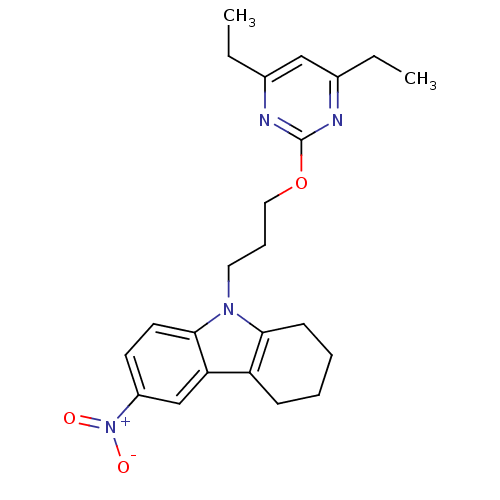
(CHEMBL2179493)Show SMILES CCc1cc(CC)nc(OCCCn2c3CCCCc3c3cc(ccc23)[N+]([O-])=O)n1 Show InChI InChI=1S/C23H28N4O3/c1-3-16-14-17(4-2)25-23(24-16)30-13-7-12-26-21-9-6-5-8-19(21)20-15-18(27(28)29)10-11-22(20)26/h10-11,14-15H,3-9,12-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397885

(CHEMBL1914885)Show SMILES COC(=O)c1ccc2n(CCCOc3nc(C)cc(C)n3)c3CCCCc3c2c1 Show InChI InChI=1S/C23H27N3O3/c1-15-13-16(2)25-23(24-15)29-12-6-11-26-20-8-5-4-7-18(20)19-14-17(22(27)28-3)9-10-21(19)26/h9-10,13-14H,4-8,11-12H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397861
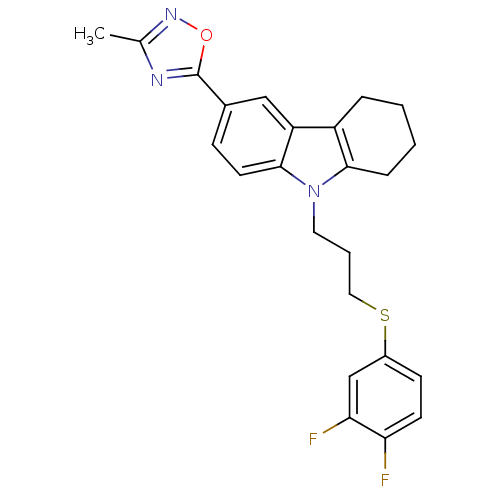
(CHEMBL2179511)Show SMILES Cc1noc(n1)-c1ccc2n(CCCSc3ccc(F)c(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3OS/c1-15-27-24(30-28-15)16-7-10-23-19(13-16)18-5-2-3-6-22(18)29(23)11-4-12-31-17-8-9-20(25)21(26)14-17/h7-10,13-14H,2-6,11-12H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397852
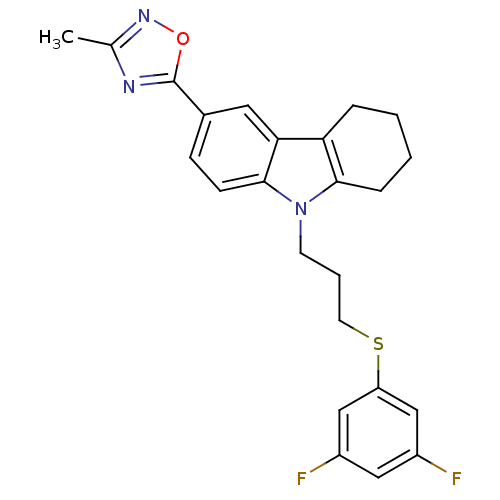
(CHEMBL2179510)Show SMILES Cc1noc(n1)-c1ccc2n(CCCSc3cc(F)cc(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3OS/c1-15-27-24(30-28-15)16-7-8-23-21(11-16)20-5-2-3-6-22(20)29(23)9-4-10-31-19-13-17(25)12-18(26)14-19/h7-8,11-14H,2-6,9-10H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397867
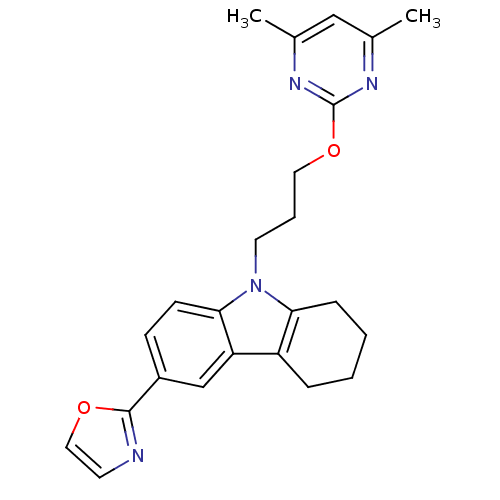
(CHEMBL2179504)Show SMILES Cc1cc(C)nc(OCCCn2c3CCCCc3c3cc(ccc23)-c2ncco2)n1 Show InChI InChI=1S/C24H26N4O2/c1-16-14-17(2)27-24(26-16)30-12-5-11-28-21-7-4-3-6-19(21)20-15-18(8-9-22(20)28)23-25-10-13-29-23/h8-10,13-15H,3-7,11-12H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397864
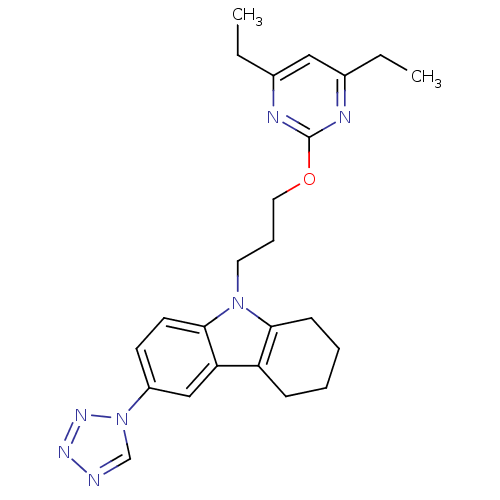
(CHEMBL2179507)Show SMILES CCc1cc(CC)nc(OCCCn2c3CCCCc3c3cc(ccc23)-n2cnnn2)n1 Show InChI InChI=1S/C24H29N7O/c1-3-17-14-18(4-2)27-24(26-17)32-13-7-12-30-22-9-6-5-8-20(22)21-15-19(10-11-23(21)30)31-16-25-28-29-31/h10-11,14-16H,3-9,12-13H2,1-2H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397863
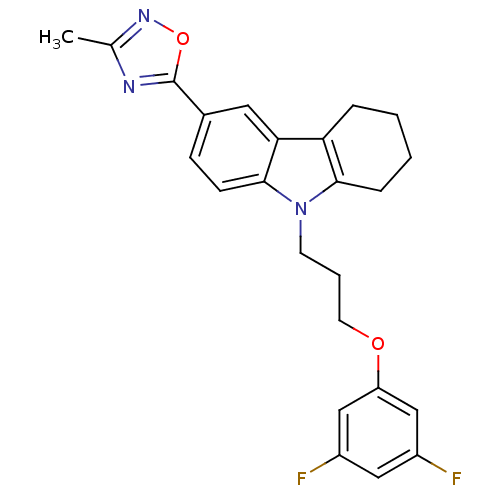
(CHEMBL2179508)Show SMILES Cc1noc(n1)-c1ccc2n(CCCOc3cc(F)cc(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3O2/c1-15-27-24(31-28-15)16-7-8-23-21(11-16)20-5-2-3-6-22(20)29(23)9-4-10-30-19-13-17(25)12-18(26)14-19/h7-8,11-14H,2-6,9-10H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397862
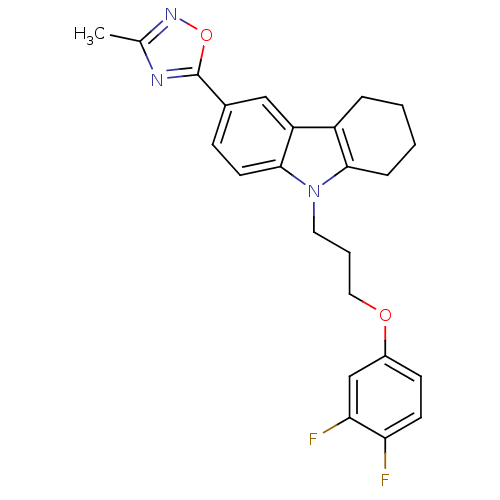
(CHEMBL2179509)Show SMILES Cc1noc(n1)-c1ccc2n(CCCOc3ccc(F)c(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3O2/c1-15-27-24(31-28-15)16-7-10-23-19(13-16)18-5-2-3-6-22(18)29(23)11-4-12-30-17-8-9-20(25)21(26)14-17/h7-10,13-14H,2-6,11-12H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397858

(CHEMBL2179514)Show SMILES Cc1noc(n1)-c1ccc2n(CCCc3nc4ccc(F)cc4[nH]3)c3CCCCc3c2c1 Show InChI InChI=1S/C25H24FN5O/c1-15-27-25(32-30-15)16-8-11-23-19(13-16)18-5-2-3-6-22(18)31(23)12-4-7-24-28-20-10-9-17(26)14-21(20)29-24/h8-11,13-14H,2-7,12H2,1H3,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397857
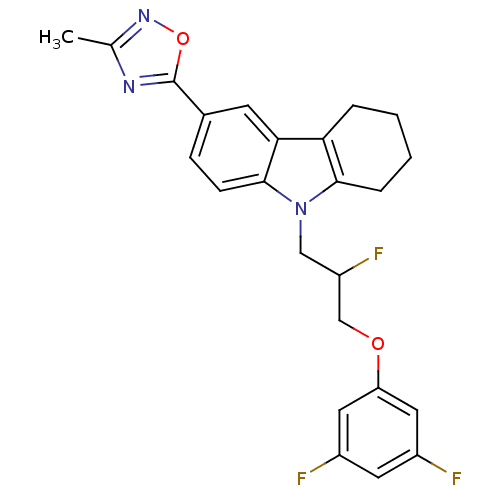
(CHEMBL2179515)Show SMILES Cc1noc(n1)-c1ccc2n(CC(F)COc3cc(F)cc(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H22F3N3O2/c1-14-28-24(32-29-14)15-6-7-23-21(8-15)20-4-2-3-5-22(20)30(23)12-18(27)13-31-19-10-16(25)9-17(26)11-19/h6-11,18H,2-5,12-13H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397852

(CHEMBL2179510)Show SMILES Cc1noc(n1)-c1ccc2n(CCCSc3cc(F)cc(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3OS/c1-15-27-24(30-28-15)16-7-8-23-21(11-16)20-5-2-3-6-22(20)29(23)9-4-10-31-19-13-17(25)12-18(26)14-19/h7-8,11-14H,2-6,9-10H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 1a entry into human HuH7.5 cells by immunoblotting |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397855
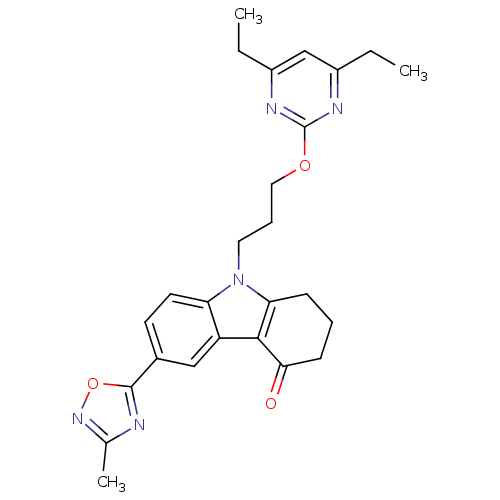
(CHEMBL2179485)Show SMILES CCc1cc(CC)nc(OCCCn2c3CCCC(=O)c3c3cc(ccc23)-c2nc(C)no2)n1 Show InChI InChI=1S/C26H29N5O3/c1-4-18-15-19(5-2)29-26(28-18)33-13-7-12-31-21-11-10-17(25-27-16(3)30-34-25)14-20(21)24-22(31)8-6-9-23(24)32/h10-11,14-15H,4-9,12-13H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10112
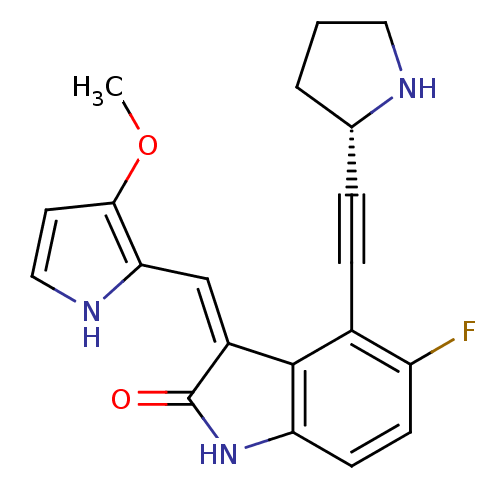
((3Z)-5-fluoro-3-[(3-methoxy-1H-pyrrol-2-yl)methyli...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H]3CCCN3)c12 |r| Show InChI InChI=1S/C20H18FN3O2/c1-26-18-8-10-23-17(18)11-14-19-13(5-4-12-3-2-9-22-12)15(21)6-7-16(19)24-20(14)25/h6-8,10-12,22-23H,2-3,9H2,1H3,(H,24,25)/b14-11-/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50305548
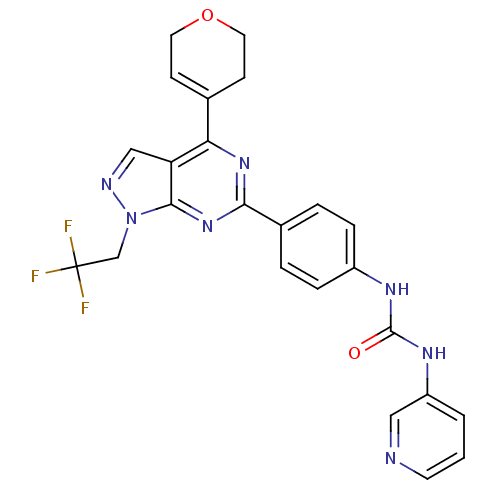
(1-(4-(4-(3,6-dihydro-2H-pyran-4-yl)-1-(2,2,2-trifl...)Show SMILES FC(F)(F)Cn1ncc2c(nc(nc12)-c1ccc(NC(=O)Nc2cccnc2)cc1)C1=CCOCC1 |t:34| Show InChI InChI=1S/C24H20F3N7O2/c25-24(26,27)14-34-22-19(13-29-34)20(15-7-10-36-11-8-15)32-21(33-22)16-3-5-17(6-4-16)30-23(35)31-18-2-1-9-28-12-18/h1-7,9,12-13H,8,10-11,14H2,(H2,30,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM10109
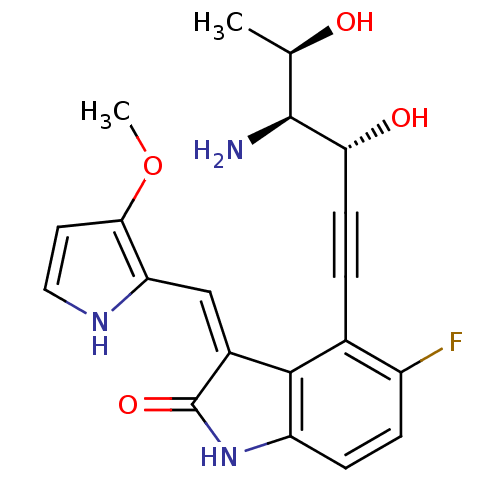
((3Z)-4-[(3R,4S,5R)-4-amino-3,5-dihydroxyhex-1-yn-1...)Show SMILES COc1cc[nH]c1\C=C1/C(=O)Nc2ccc(F)c(C#C[C@@H](O)[C@@H](N)[C@@H](C)O)c12 |r| Show InChI InChI=1S/C20H20FN3O4/c1-10(25)19(22)16(26)6-3-11-13(21)4-5-14-18(11)12(20(27)24-14)9-15-17(28-2)7-8-23-15/h4-5,7-10,16,19,23,25-26H,22H2,1-2H3,(H,24,27)/b12-9-/t10-,16-,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50044262

(CHEMBL3314084)Show SMILES Clc1cccc(Cl)c1N1C(=O)\C(=C\c2coc3ccccc3c2=O)c2ccccc12 Show InChI InChI=1S/C24H13Cl2NO3/c25-18-8-5-9-19(26)22(18)27-20-10-3-1-6-15(20)17(24(27)29)12-14-13-30-21-11-4-2-7-16(21)23(14)28/h1-13H/b17-12+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as decrease in prostaglandin production using arachidonic acid as substrate incubated with enzyme for 10 mins prio... |
Eur J Med Chem 77: 185-92 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.003
BindingDB Entry DOI: 10.7270/Q2319XH6 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397856

(CHEMBL2179106)Show SMILES Cc1noc(n1)-c1ccc2n(CCCSc3cc(F)cc(F)c3)c3CCCC(=O)c3c2c1 Show InChI InChI=1S/C24H21F2N3O2S/c1-14-27-24(31-28-14)15-6-7-20-19(10-15)23-21(4-2-5-22(23)30)29(20)8-3-9-32-18-12-16(25)11-17(26)13-18/h6-7,10-13H,2-5,8-9H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397860
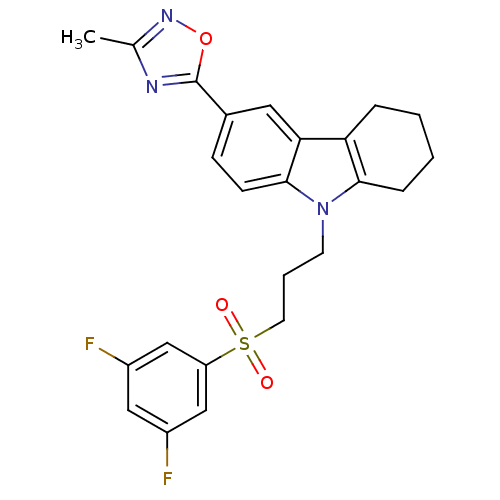
(CHEMBL2179512)Show SMILES Cc1noc(n1)-c1ccc2n(CCCS(=O)(=O)c3cc(F)cc(F)c3)c3CCCCc3c2c1 Show InChI InChI=1S/C24H23F2N3O3S/c1-15-27-24(32-28-15)16-7-8-23-21(11-16)20-5-2-3-6-22(20)29(23)9-4-10-33(30,31)19-13-17(25)12-18(26)14-19/h7-8,11-14H,2-6,9-10H2,1H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50404086
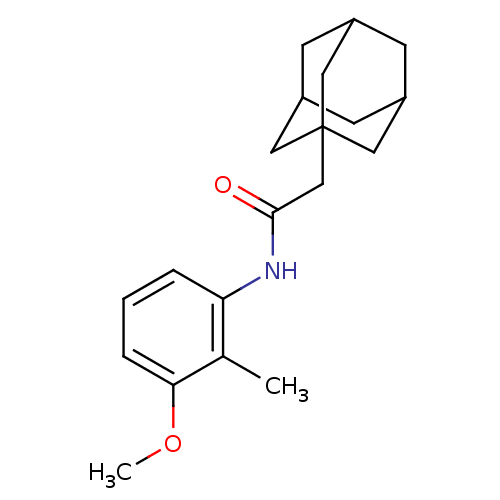
(CHEMBL341226)Show SMILES COc1cccc(NC(=O)CC23CC4CC(CC(C4)C2)C3)c1C |TLB:20:11:18:15.14.16,THB:19:11:14:17.18.16,19:17:11.12.20:14,20:15:11.12.19:18| Show InChI InChI=1S/C20H27NO2/c1-13-17(4-3-5-18(13)23-2)21-19(22)12-20-9-14-6-15(10-20)8-16(7-14)11-20/h3-5,14-16H,6-12H2,1-2H3,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50235342

(CHEMBL319467 | NU-6102)Show SMILES NS(=O)(=O)c1ccc(Nc2nc(OCC3CCCCC3)c3nc[nH]c3n2)cc1 Show InChI InChI=1S/C18H22N6O3S/c19-28(25,26)14-8-6-13(7-9-14)22-18-23-16-15(20-11-21-16)17(24-18)27-10-12-4-2-1-3-5-12/h6-9,11-12H,1-5,10H2,(H2,19,25,26)(H2,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| PDB
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Scavenger receptor class B member 1
(Homo sapiens (Human)) | BDBM50397874
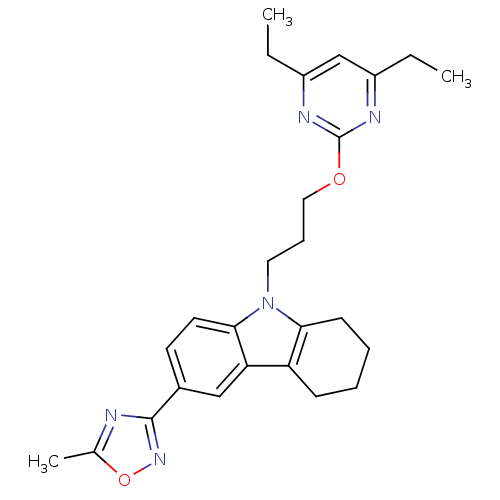
(CHEMBL2179497)Show SMILES CCc1cc(CC)nc(OCCCn2c3CCCCc3c3cc(ccc23)-c2noc(C)n2)n1 Show InChI InChI=1S/C26H31N5O2/c1-4-19-16-20(5-2)29-26(28-19)32-14-8-13-31-23-10-7-6-9-21(23)22-15-18(11-12-24(22)31)25-27-17(3)33-30-25/h11-12,15-16H,4-10,13-14H2,1-3H3 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
iTherX Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SRB1-mediated Hepatitis C virus genotype 2a entry into human HuH7.5 cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 22: 4955-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.038
BindingDB Entry DOI: 10.7270/Q20R9QH1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50132450

(3-[1-(5-Methoxy-1-methyl-1H-indol-3-yl)-meth-(Z)-y...)Show SMILES COc1ccc2n(C)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1 Show InChI InChI=1S/C19H17N3O4S/c1-22-10-11(14-8-12(26-2)3-6-18(14)22)7-16-15-9-13(27(20,24)25)4-5-17(15)21-19(16)23/h3-10H,1-2H3,(H,21,23)(H2,20,24,25)/b16-7- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Inhibition of spleen tyrosine kinase (unknown origin) |
Eur J Med Chem 67: 434-46 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.070
BindingDB Entry DOI: 10.7270/Q2Z039JB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50613598
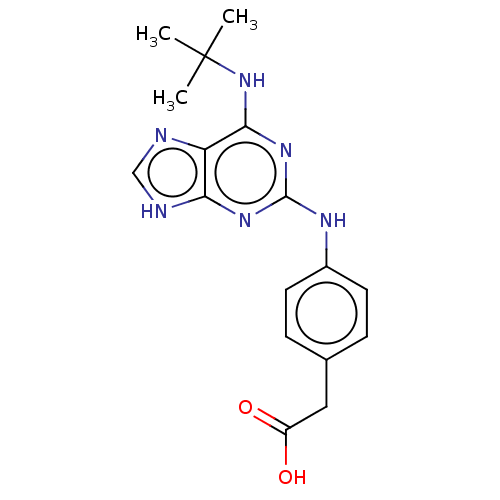
(CHEMBL5283028)Show SMILES CC(C)(C)Nc1nc(Nc2ccc(CC(O)=O)cc2)nc2[nH]cnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50613599
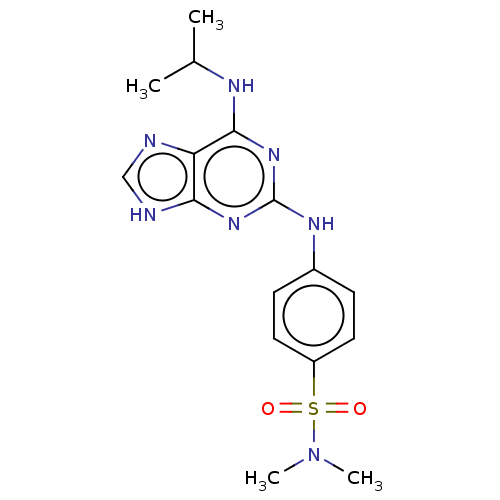
(CHEMBL5273296)Show SMILES CC(C)Nc1nc(Nc2ccc(cc2)S(=O)(=O)N(C)C)nc2[nH]cnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50613600
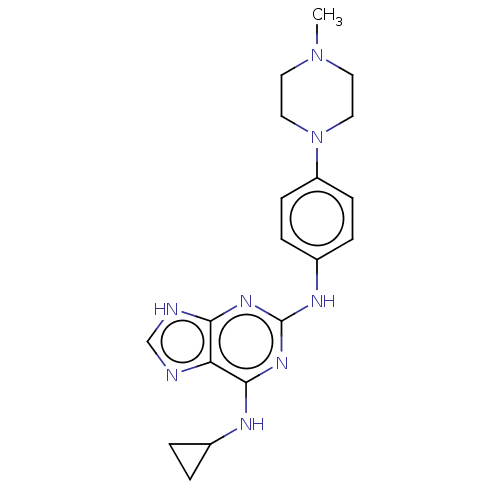
(CHEMBL5285207)Show SMILES CN1CCN(CC1)c1ccc(Nc2nc(NC3CC3)c3nc[nH]c3n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50613601
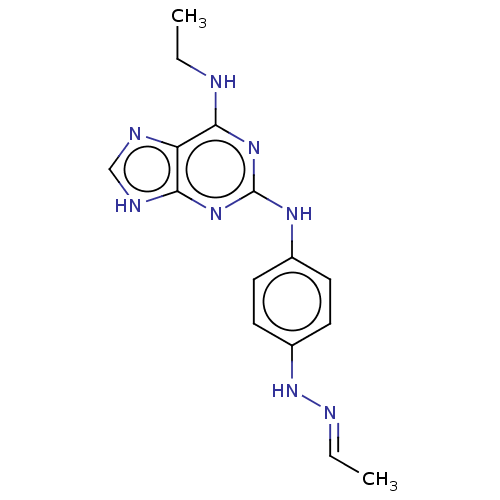
(CHEMBL5270442) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50613602
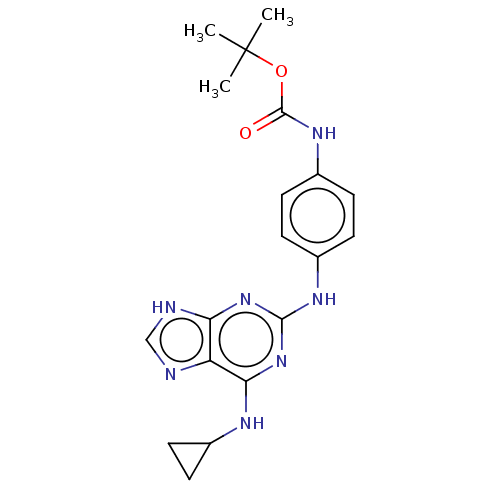
(CHEMBL5268541)Show SMILES CC(C)(C)OC(=O)Nc1ccc(Nc2nc(NC3CC3)c3nc[nH]c3n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126713
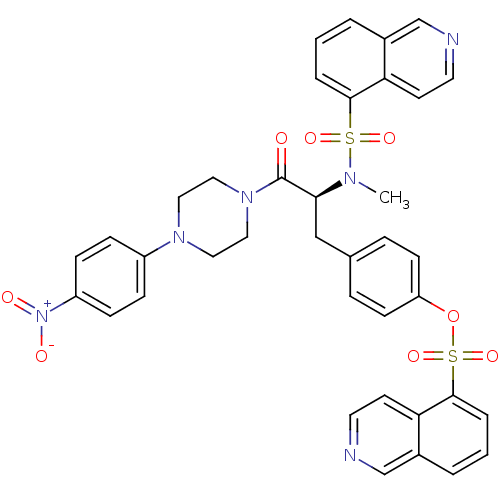
(CHEMBL282173 | Isoquinoline-5-sulfonic acid 4-{(S)...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(CC1)c1ccc(cc1)[N+]([O-])=O)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C38H34N6O8S2/c1-41(53(48,49)36-6-2-4-28-25-39-18-16-33(28)36)35(38(45)43-22-20-42(21-23-43)30-10-12-31(13-11-30)44(46)47)24-27-8-14-32(15-9-27)52-54(50,51)37-7-3-5-29-26-40-19-17-34(29)37/h2-19,25-26,35H,20-24H2,1H3/t35-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50126702
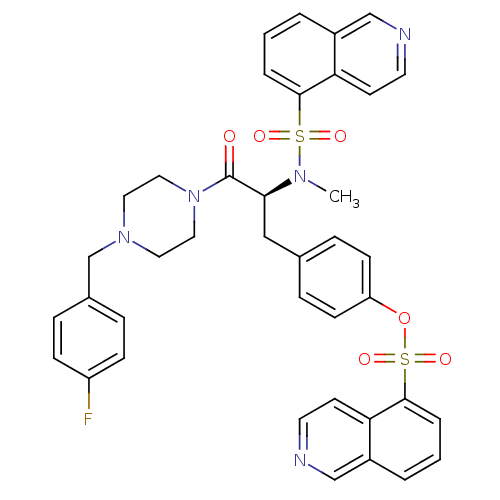
(CHEMBL282807 | Isoquinoline-5-sulfonic acid 4-{(S)...)Show SMILES CN([C@@H](Cc1ccc(OS(=O)(=O)c2cccc3cnccc23)cc1)C(=O)N1CCN(Cc2ccc(F)cc2)CC1)S(=O)(=O)c1cccc2cnccc12 Show InChI InChI=1S/C39H36FN5O6S2/c1-43(52(47,48)37-6-2-4-30-25-41-18-16-34(30)37)36(39(46)45-22-20-44(21-23-45)27-29-8-12-32(40)13-9-29)24-28-10-14-33(15-11-28)51-53(49,50)38-7-3-5-31-26-42-19-17-35(31)38/h2-19,25-26,36H,20-24,27H2,1H3/t36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University
Curated by ChEMBL
| Assay Description
Antagonist activity at P2X7 receptor (unknown origin) |
Bioorg Med Chem 22: 54-88 (2014)
Article DOI: 10.1016/j.bmc.2013.10.054
BindingDB Entry DOI: 10.7270/Q27M0BX7 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50044264
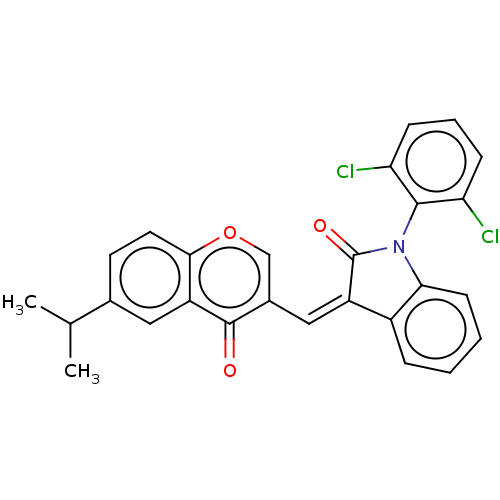
(CHEMBL3314086)Show SMILES CC(C)c1ccc2occ(\C=C3\C(=O)N(c4ccccc34)c3c(Cl)cccc3Cl)c(=O)c2c1 Show InChI InChI=1S/C27H19Cl2NO3/c1-15(2)16-10-11-24-20(12-16)26(31)17(14-33-24)13-19-18-6-3-4-9-23(18)30(27(19)32)25-21(28)7-5-8-22(25)29/h3-15H,1-2H3/b19-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX-2 assessed as decrease in prostaglandin production using arachidonic acid as substrate incubated with enzyme for ... |
Eur J Med Chem 77: 185-92 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.003
BindingDB Entry DOI: 10.7270/Q2319XH6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data