 Found 686 hits with Last Name = 'kawasaki' and Initial = 'a'
Found 686 hits with Last Name = 'kawasaki' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587520

(US11535596, Compound CNS27-D2)Show SMILES COCCCN1CCC23CCCCC2C1Cc1ccc(OCC(F)(F)F)cc31 |TLB:4:5:13:27.16.15,THB:26:27:13:5.7.6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587509
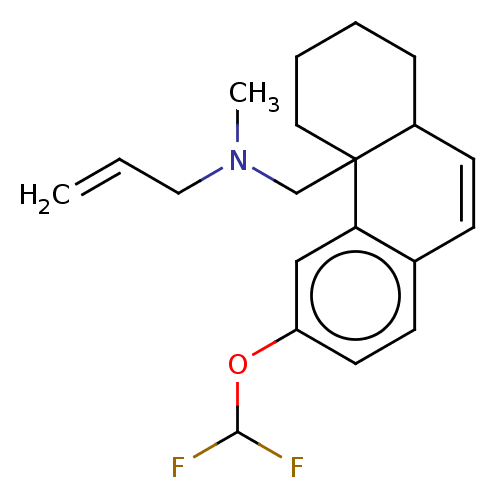
(US11535596, Compound CNS23-D2)Show SMILES CN(CC=C)CC12CCCCC1C=Cc1ccc(OC(F)F)cc21 |c:13| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587505
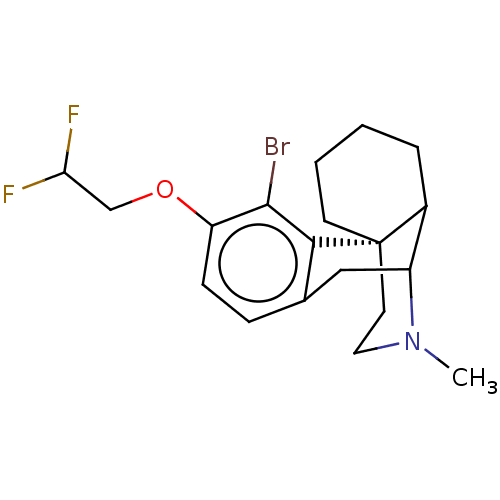
(US11535596, Compound CNS6-A1-D2)Show SMILES CN1CC[C@@]23CCCCC2C1Cc1ccc(OCC(F)F)c(Br)c31 |r,THB:21:23:9:1.2.3,13:12:9:1.2.3| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 15.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587511

(US11535596, Compound CNS25)Show SMILES COCCN(C)CC12CCCCC1(C)C=Cc1ccc(C)cc21 |c:15| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587508

(US11535596, Compound CNS22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587516

(US11535596, Compound CNS1-D5)Show SMILES Oc1ccc2CC3NCCC4(CCCCC34)c2c1 |THB:17:16:15:7.9.8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587515
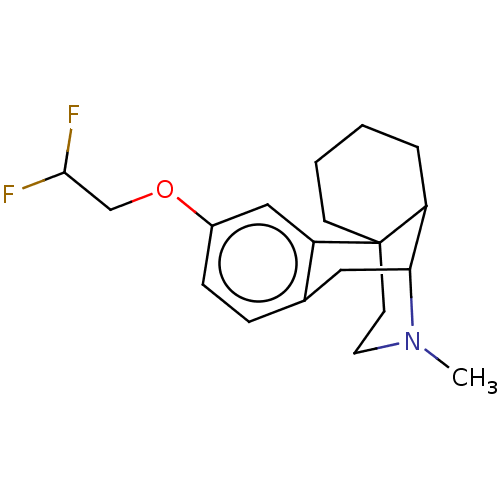
(US11535596, Compound CNS1-D2)Show SMILES CN1CCC23CCCCC2C1Cc1ccc(OCC(F)F)cc31 |THB:0:1:9:22.12.11,21:22:9:1.3.2| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 40.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587513
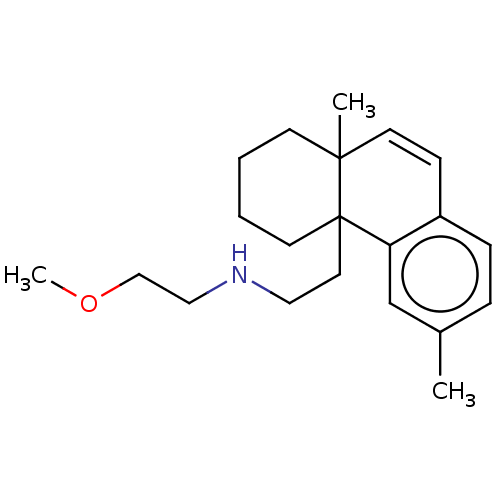
(US11535596, Compound CNS26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 55.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587521
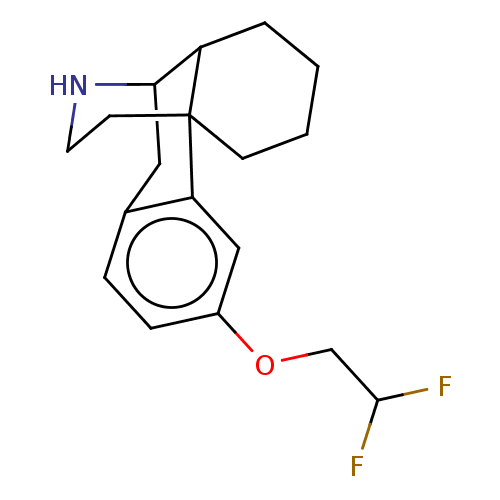
(US11535596, Compound N-desmethyl CNS1-D2)Show SMILES FC(F)COc1ccc2CC3NCCC4(CCCCC34)c2c1 |THB:21:20:19:11.13.12| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM213826

(Dextromethorphan | US11535596, Compound Dextrometh...)Show SMILES COc1ccc2C[C@@H]3[C@H]4CCCC[C@]4(CCN3C)c2c1 |THB:17:16:8:18.6.5| Show InChI InChI=1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15-,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 96.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587504
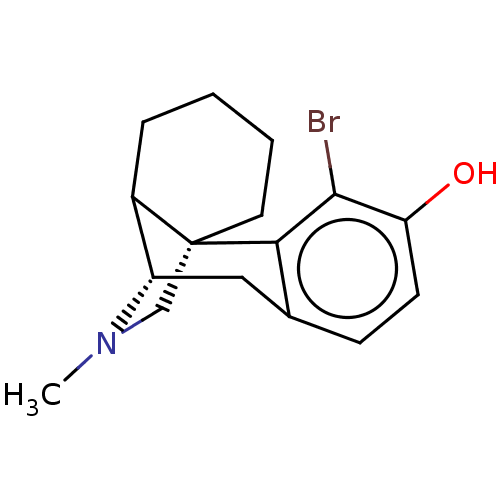
(US11535596, Compound CNS6)Show SMILES CN1C[C@@]23CCCCC2[C@@H]1Cc1ccc(O)c(Br)c31 |TLB:0:1:8:18.11.10| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50001000
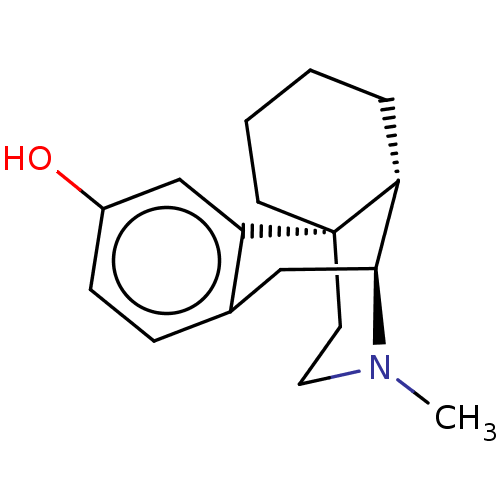
(CHEBI:29133 | DEXTRORPHAN | US11535596, Compound D...)Show SMILES [H][C@]12Cc3ccc(O)cc3[C@@]3(CCCC[C@]13[H])CCN2C |r,THB:20:19:15:2.9.3| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM587503

(US11535596, Compound Dimemorfan (DIM))Show SMILES CN1CC[C@@]23CCCC[C@@H]2[C@@H]1Cc1ccc(C)cc31 |THB:8:9:18.12.11:3.2.1| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
UniChem
| US Patent
| 371 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM50001000

(CHEBI:29133 | DEXTRORPHAN | US11535596, Compound D...)Show SMILES [H][C@]12Cc3ccc(O)cc3[C@@]3(CCCC[C@]13[H])CCN2C |r,THB:20:19:15:2.9.3| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM587521

(US11535596, Compound N-desmethyl CNS1-D2)Show SMILES FC(F)COc1ccc2CC3NCCC4(CCCCC34)c2c1 |THB:21:20:19:11.13.12| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM587508

(US11535596, Compound CNS22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM213826

(Dextromethorphan | US11535596, Compound Dextrometh...)Show SMILES COc1ccc2C[C@@H]3[C@H]4CCCC[C@]4(CCN3C)c2c1 |THB:17:16:8:18.6.5| Show InChI InChI=1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15-,17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM587516

(US11535596, Compound CNS1-D5)Show SMILES Oc1ccc2CC3NCCC4(CCCCC34)c2c1 |THB:17:16:15:7.9.8| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM587511

(US11535596, Compound CNS25)Show SMILES COCCN(C)CC12CCCCC1(C)C=Cc1ccc(C)cc21 |c:15| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1
(Homo sapiens (Human)) | BDBM587520

(US11535596, Compound CNS27-D2)Show SMILES COCCCN1CCC23CCCCC2C1Cc1ccc(OCC(F)(F)F)cc31 |TLB:4:5:13:27.16.15,THB:26:27:13:5.7.6| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| US Patent
| >2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro binding assays for the phencyclidine site of NMDA-type glutamate receptors, and for sigma-1 receptors, both of which are known to be bound b... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2086945 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010705
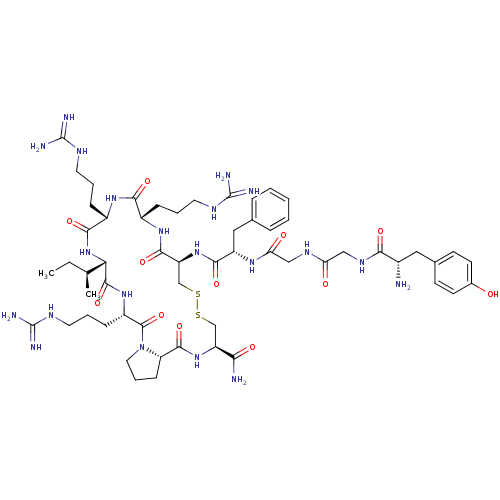
(CHEMBL385759 | H-Tyr-Gly-Gly-Phe-Cys-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C57H89N21O12S2/c1-3-31(2)45-53(89)74-38(15-9-23-68-57(64)65)54(90)78-24-10-16-42(78)52(88)75-40(46(59)82)29-91-92-30-41(51(87)73-36(13-7-21-66-55(60)61)48(84)72-37(49(85)77-45)14-8-22-67-56(62)63)76-50(86)39(26-32-11-5-4-6-12-32)71-44(81)28-69-43(80)27-70-47(83)35(58)25-33-17-19-34(79)20-18-33/h4-6,11-12,17-20,31,35-42,45,79H,3,7-10,13-16,21-30,58H2,1-2H3,(H2,59,82)(H,69,80)(H,70,83)(H,71,81)(H,72,84)(H,73,87)(H,74,89)(H,75,88)(H,76,86)(H,77,85)(H4,60,61,66)(H4,62,63,67)(H4,64,65,68)/t31-,35-,36-,37-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 in guinea pig brain homogenate using [3H]- PL-17 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040125
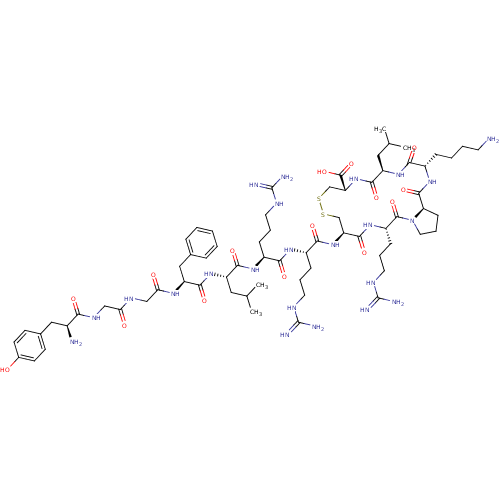
(CHEMBL438274 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-c(Cys-A...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O Show InChI InChI=1S/C69H111N23O15S2/c1-38(2)30-48(89-62(102)50(33-40-14-6-5-7-15-40)83-55(95)35-81-54(94)34-82-56(96)43(71)32-41-21-23-42(93)24-22-41)60(100)85-45(17-10-26-78-67(72)73)57(97)84-46(18-11-27-79-68(74)75)59(99)90-51-36-108-109-37-52(66(106)107)91-61(101)49(31-39(3)4)88-58(98)44(16-8-9-25-70)86-64(104)53-20-13-29-92(53)65(105)47(87-63(51)103)19-12-28-80-69(76)77/h5-7,14-15,21-24,38-39,43-53,93H,8-13,16-20,25-37,70-71H2,1-4H3,(H,81,94)(H,82,96)(H,83,95)(H,84,97)(H,85,100)(H,86,104)(H,87,103)(H,88,98)(H,89,102)(H,90,99)(H,91,101)(H,106,107)(H4,72,73,78)(H4,74,75,79)(H4,76,77,80)/t43-,44-,45-,46-,47-,48-,49+,50-,51-,52+,53+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040126

(CHEMBL265241 | Tyr-Gly-Gly-Phe-leu-c(Cys-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C58H92N18O13S2/c1-5-33(4)47-55(87)71-38(16-11-23-65-57(61)62)49(81)74-44(54(86)72-40(56(88)89)15-9-10-22-59)31-91-90-30-43(53(85)70-39(50(82)76-47)17-12-24-66-58(63)64)75-51(83)41(25-32(2)3)73-52(84)42(27-34-13-7-6-8-14-34)69-46(79)29-67-45(78)28-68-48(80)37(60)26-35-18-20-36(77)21-19-35/h6-8,13-14,18-21,32-33,37-44,47,77H,5,9-12,15-17,22-31,59-60H2,1-4H3,(H,67,78)(H,68,80)(H,69,79)(H,70,85)(H,71,87)(H,72,86)(H,73,84)(H,74,81)(H,75,83)(H,76,82)(H,88,89)(H4,61,62,65)(H4,63,64,66)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010703
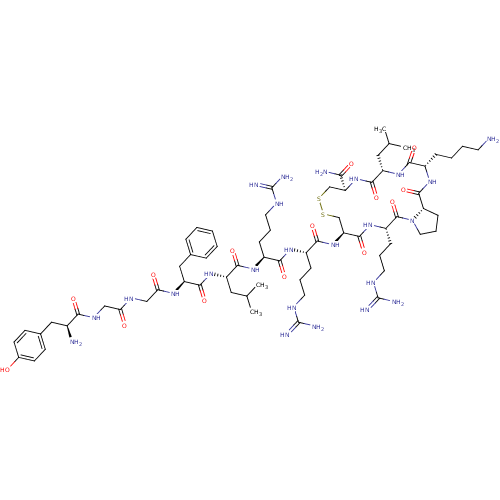
(CHEMBL443068 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Cys-A...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C69H112N24O14S2/c1-38(2)30-48(90-63(104)50(33-40-14-6-5-7-15-40)84-55(96)35-82-54(95)34-83-57(98)43(71)32-41-21-23-42(94)24-22-41)61(102)86-45(17-10-26-79-67(73)74)58(99)85-46(18-11-27-80-68(75)76)60(101)92-52-37-109-108-36-51(56(72)97)91-62(103)49(31-39(3)4)89-59(100)44(16-8-9-25-70)87-65(106)53-20-13-29-93(53)66(107)47(88-64(52)105)19-12-28-81-69(77)78/h5-7,14-15,21-24,38-39,43-53,94H,8-13,16-20,25-37,70-71H2,1-4H3,(H2,72,97)(H,82,95)(H,83,98)(H,84,96)(H,85,99)(H,86,102)(H,87,106)(H,88,105)(H,89,100)(H,90,104)(H,91,103)(H,92,101)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040123
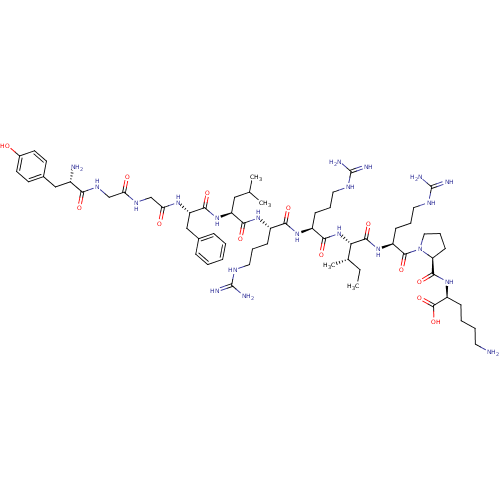
(CHEMBL438223 | HTry-Gly-Gly-Phe-Leu-Arg-Arg-lle-Ar...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C63H103N21O13/c1-5-37(4)51(58(94)80-44(20-13-29-74-63(70)71)59(95)84-30-14-21-48(84)57(93)81-45(60(96)97)17-9-10-26-64)83-54(90)43(19-12-28-73-62(68)69)78-53(89)42(18-11-27-72-61(66)67)79-55(91)46(31-36(2)3)82-56(92)47(33-38-15-7-6-8-16-38)77-50(87)35-75-49(86)34-76-52(88)41(65)32-39-22-24-40(85)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,85H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H,75,86)(H,76,88)(H,77,87)(H,78,89)(H,79,91)(H,80,94)(H,81,93)(H,82,92)(H,83,90)(H,96,97)(H4,66,67,72)(H4,68,69,73)(H4,70,71,74)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010704

(CHEMBL216640 | Dyn A(1-11)-NH2 | Dynorphin A analo...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C63H104N22O12/c1-5-37(4)51(59(96)82-45(20-13-29-75-63(71)72)60(97)85-30-14-21-48(85)58(95)79-42(52(66)89)17-9-10-26-64)84-55(92)44(19-12-28-74-62(69)70)80-54(91)43(18-11-27-73-61(67)68)81-56(93)46(31-36(2)3)83-57(94)47(33-38-15-7-6-8-16-38)78-50(88)35-76-49(87)34-77-53(90)41(65)32-39-22-24-40(86)25-23-39/h6-8,15-16,22-25,36-37,41-48,51,86H,5,9-14,17-21,26-35,64-65H2,1-4H3,(H2,66,89)(H,76,87)(H,77,90)(H,78,88)(H,79,95)(H,80,91)(H,81,93)(H,82,96)(H,83,94)(H,84,92)(H4,67,68,73)(H4,69,70,74)(H4,71,72,75)/t37-,41-,42-,43-,44-,45-,46-,47-,48-,51-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50040126

(CHEMBL265241 | Tyr-Gly-Gly-Phe-leu-c(Cys-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C58H92N18O13S2/c1-5-33(4)47-55(87)71-38(16-11-23-65-57(61)62)49(81)74-44(54(86)72-40(56(88)89)15-9-10-22-59)31-91-90-30-43(53(85)70-39(50(82)76-47)17-12-24-66-58(63)64)75-51(83)41(25-32(2)3)73-52(84)42(27-34-13-7-6-8-14-34)69-46(79)29-67-45(78)28-68-48(80)37(60)26-35-18-20-36(77)21-19-35/h6-8,13-14,18-21,32-33,37-44,47,77H,5,9-12,15-17,22-31,59-60H2,1-4H3,(H,67,78)(H,68,80)(H,69,79)(H,70,85)(H,71,87)(H,72,86)(H,73,84)(H,74,81)(H,75,83)(H,76,82)(H,88,89)(H4,61,62,65)(H4,63,64,66)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PL-17 binding to mu opioid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010701
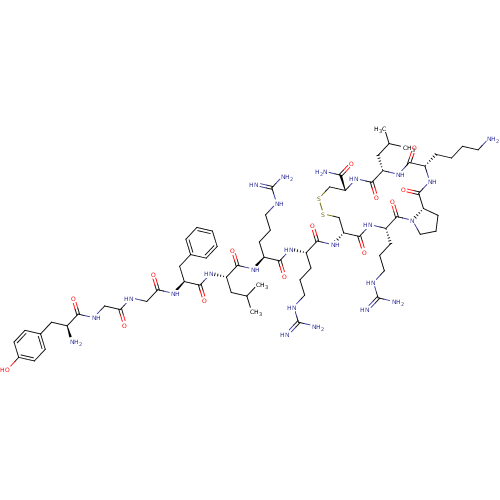
(CHEMBL410616 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-D-Cys...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C69H112N24O14S2/c1-38(2)30-48(90-63(104)50(33-40-14-6-5-7-15-40)84-55(96)35-82-54(95)34-83-57(98)43(71)32-41-21-23-42(94)24-22-41)61(102)86-45(17-10-26-79-67(73)74)58(99)85-46(18-11-27-80-68(75)76)60(101)92-52-37-109-108-36-51(56(72)97)91-62(103)49(31-39(3)4)89-59(100)44(16-8-9-25-70)87-65(106)53-20-13-29-93(53)66(107)47(88-64(52)105)19-12-28-81-69(77)78/h5-7,14-15,21-24,38-39,43-53,94H,8-13,16-20,25-37,70-71H2,1-4H3,(H2,72,97)(H,82,95)(H,83,98)(H,84,96)(H,85,99)(H,86,102)(H,87,106)(H,88,105)(H,89,100)(H,90,104)(H,91,103)(H,92,101)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,44-,45-,46-,47-,48-,49-,50-,51+,52+,53-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM224024

(BDBM50241435 | Dynorphin A (1-13) | YGGFLRRXRPKLK)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#8])=O Show InChI InChI=1S/C75H126N24O15/c1-7-45(6)61(70(111)94-53(25-17-35-87-75(83)84)71(112)99-36-18-26-58(99)69(110)93-50(21-11-13-31-76)64(105)96-56(38-44(4)5)67(108)95-54(72(113)114)22-12-14-32-77)98-65(106)52(24-16-34-86-74(81)82)91-63(104)51(23-15-33-85-73(79)80)92-66(107)55(37-43(2)3)97-68(109)57(40-46-19-9-8-10-20-46)90-60(102)42-88-59(101)41-89-62(103)49(78)39-47-27-29-48(100)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,100H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H,88,101)(H,89,103)(H,90,102)(H,91,104)(H,92,107)(H,93,110)(H,94,111)(H,95,108)(H,96,105)(H,97,109)(H,98,106)(H,113,114)(H4,79,80,85)(H4,81,82,86)(H4,83,84,87)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50002184

(CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(N)=O Show InChI InChI=1S/C75H127N25O14/c1-7-45(6)61(71(113)96-54(25-17-35-88-75(84)85)72(114)100-36-18-26-58(100)70(112)95-51(22-12-14-32-77)65(107)97-55(37-43(2)3)67(109)92-50(62(79)104)21-11-13-31-76)99-66(108)53(24-16-34-87-74(82)83)93-64(106)52(23-15-33-86-73(80)81)94-68(110)56(38-44(4)5)98-69(111)57(40-46-19-9-8-10-20-46)91-60(103)42-89-59(102)41-90-63(105)49(78)39-47-27-29-48(101)30-28-47/h8-10,19-20,27-30,43-45,49-58,61,101H,7,11-18,21-26,31-42,76-78H2,1-6H3,(H2,79,104)(H,89,102)(H,90,105)(H,91,103)(H,92,109)(H,93,106)(H,94,110)(H,95,112)(H,96,113)(H,97,107)(H,98,111)(H,99,108)(H4,80,81,86)(H4,82,83,87)(H4,84,85,88)/t45-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,61-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.114 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040131

(CHEMBL409793 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-c((D-Cy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C69H112N24O15S2/c1-39(2)32-49(90-61(102)50(34-40-14-4-3-5-15-40)84-55(96)36-82-54(95)35-83-56(97)43(72)33-41-22-24-42(94)25-23-41)60(101)86-45(18-10-28-79-67(73)74)57(98)85-46(19-11-29-80-68(75)76)59(100)91-51-37-109-110-38-52(63(104)89-48(66(107)108)17-7-9-27-71)92-58(99)44(16-6-8-26-70)87-64(105)53-21-13-31-93(53)65(106)47(88-62(51)103)20-12-30-81-69(77)78/h3-5,14-15,22-25,39,43-53,94H,6-13,16-21,26-38,70-72H2,1-2H3,(H,82,95)(H,83,97)(H,84,96)(H,85,98)(H,86,101)(H,87,105)(H,88,103)(H,89,104)(H,90,102)(H,91,100)(H,92,99)(H,107,108)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,44+,45-,46-,47+,48-,49-,50-,51+,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.116 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040132
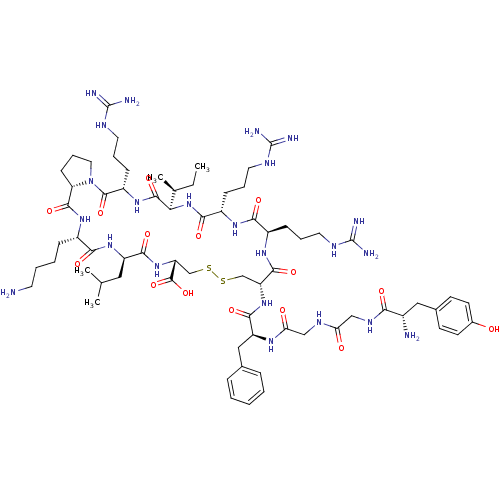
(CHEMBL411196 | Tyr-Gly-Gly-Phe-c(Cys-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C69H111N23O15S2/c1-5-39(4)55-64(104)87-47(20-13-29-80-69(76)77)65(105)92-30-14-21-52(92)63(103)86-44(17-9-10-26-70)58(98)88-48(31-38(2)3)60(100)90-51(66(106)107)37-109-108-36-50(62(102)85-45(18-11-27-78-67(72)73)57(97)84-46(59(99)91-55)19-12-28-79-68(74)75)89-61(101)49(33-40-15-7-6-8-16-40)83-54(95)35-81-53(94)34-82-56(96)43(71)32-41-22-24-42(93)25-23-41/h6-8,15-16,22-25,38-39,43-52,55,93H,5,9-14,17-21,26-37,70-71H2,1-4H3,(H,81,94)(H,82,96)(H,83,95)(H,84,97)(H,85,102)(H,86,103)(H,87,104)(H,88,98)(H,89,101)(H,90,100)(H,91,99)(H,106,107)(H4,72,73,78)(H4,74,75,79)(H4,76,77,80)/t39-,43-,44-,45+,46-,47-,48+,49-,50+,51+,52-,55+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50063895
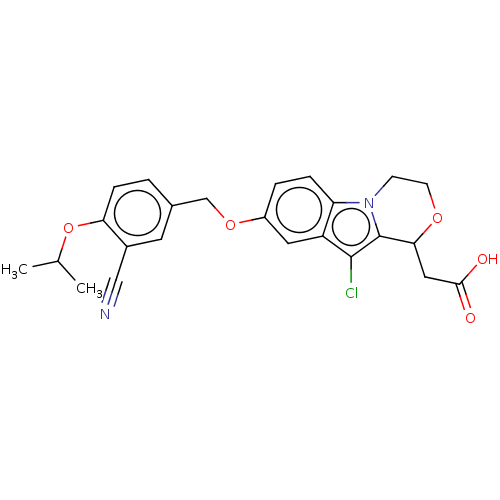
(CHEMBL3403631)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CCOC(CC(O)=O)c4c(Cl)c3c2)cc1C#N Show InChI InChI=1S/C24H23ClN2O5/c1-14(2)32-20-6-3-15(9-16(20)12-26)13-31-17-4-5-19-18(10-17)23(25)24-21(11-22(28)29)30-8-7-27(19)24/h3-6,9-10,14,21H,7-8,11,13H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor assessed as change in cAMP level by homogeneous time resolved fluorescence cyclase assay |
Bioorg Med Chem Lett 25: 659-63 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.089
BindingDB Entry DOI: 10.7270/Q25T3N5P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50063895

(CHEMBL3403631)Show SMILES CC(C)Oc1ccc(COc2ccc3n4CCOC(CC(O)=O)c4c(Cl)c3c2)cc1C#N Show InChI InChI=1S/C24H23ClN2O5/c1-14(2)32-20-6-3-15(9-16(20)12-26)13-31-17-4-5-19-18(10-17)23(25)24-21(11-22(28)29)30-8-7-27(19)24/h3-6,9-10,14,21H,7-8,11,13H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P1 receptor assessed as change in cAMP level by homogeneous time resolved fluorescence cyclase assay |
Bioorg Med Chem Lett 25: 659-63 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.089
BindingDB Entry DOI: 10.7270/Q25T3N5P |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010702
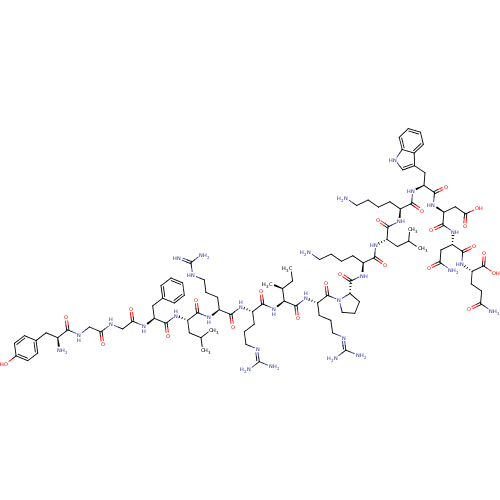
(CHEMBL411557 | DYNORPHIN(1-17)-OH | Dynorphin A (1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:38.46,19.26,4.4,2.2,88.90,128.132,144.148,70.71,105.107,wD:57.58,30.34,8.15,84.87,97.99,114.116,136.140,(27.24,-21.93,;25.83,-21.28,;25.7,-19.76,;26.97,-18.87,;24.28,-19.11,;24.14,-17.56,;25.39,-16.68,;26.8,-17.34,;25.25,-15.13,;23.87,-14.49,;23.73,-12.97,;22.32,-12.32,;22.18,-10.79,;20.78,-10.14,;19.51,-11.03,;20.63,-8.6,;26.5,-14.25,;26.41,-12.7,;25.05,-12.02,;27.68,-11.87,;29.07,-12.56,;30.37,-11.71,;31.74,-12.4,;33.01,-11.53,;34.37,-12.24,;34.46,-13.77,;35.67,-11.39,;27.59,-10.31,;28.87,-9.48,;30.26,-10.18,;28.78,-7.92,;27.42,-7.24,;26.12,-8.09,;24.76,-7.41,;26.21,-9.64,;30.05,-7.09,;29.97,-5.53,;28.6,-4.85,;31.24,-4.7,;32.63,-5.4,;32.72,-6.92,;31.45,-7.77,;31.55,-9.31,;32.93,-9.99,;34.21,-9.14,;34.11,-7.6,;31.15,-3.14,;32.43,-2.31,;33.81,-3.01,;32.34,-.76,;33.61,.08,;33.52,1.63,;32.16,2.32,;34.8,2.47,;34.71,4.01,;36,4.86,;37.37,4.17,;35.91,6.4,;37.19,7.24,;34.53,7.1,;33.23,6.24,;33.34,4.69,;32.06,3.84,;30.68,4.52,;29.39,3.71,;30.58,6.06,;31.86,6.91,;23.04,-19.99,;21.65,-19.34,;23.18,-21.52,;21.92,-22.42,;22.06,-23.97,;20.82,-24.84,;19.43,-24.2,;18.16,-25.1,;16.78,-24.46,;16.64,-22.94,;15.51,-25.35,;20.12,-21.58,;18.85,-22.48,;19.94,-19.61,;21.09,-18.4,;20.31,-16.91,;18.66,-17.19,;18.69,-18.75,;17.33,-19.47,;17.28,-21.01,;16.02,-18.66,;14.66,-19.38,;14.61,-20.92,;13.25,-21.64,;13.2,-23.18,;11.84,-23.91,;11.79,-25.45,;13.36,-18.57,;13.41,-17.03,;12,-19.29,;10.69,-18.48,;10.74,-16.94,;9.44,-16.12,;9.49,-14.58,;8.08,-16.85,;9.33,-19.2,;9.28,-20.74,;8.03,-18.39,;6.67,-19.11,;6.61,-20.65,;5.26,-21.37,;5.2,-22.91,;3.85,-23.64,;3.79,-25.18,;5.36,-18.3,;5.41,-16.76,;4,-19.02,;2.69,-18.21,;2.75,-16.67,;1.44,-15.85,;.01,-16.43,;-.98,-15.25,;-.16,-13.95,;-.59,-12.47,;.48,-11.36,;1.97,-11.73,;2.4,-13.21,;1.33,-14.32,;1.34,-18.93,;1.28,-20.47,;.03,-18.12,;-1.33,-18.84,;-1.38,-20.38,;-2.74,-21.1,;-4.05,-20.29,;-2.79,-22.64,;-2.64,-18.03,;-2.59,-16.49,;-4,-18.75,;-5.3,-17.94,;-5.25,-16.4,;-6.56,-15.58,;-7.92,-16.31,;-6.51,-14.04,;-6.66,-18.66,;-6.71,-20.2,;-7.97,-17.85,;-9.33,-18.57,;-9.38,-20.11,;-10.74,-20.83,;-10.79,-22.37,;-9.48,-23.19,;-12.15,-23.1,;-10.63,-17.76,;-11.99,-18.48,;-10.58,-16.22,)| Show InChI InChI=1S/C99H155N31O23/c1-7-55(6)81(129-86(142)66(28-18-40-112-98(107)108)118-83(139)65(27-17-39-111-97(105)106)120-88(144)70(44-54(4)5)125-89(145)71(46-56-21-9-8-10-22-56)117-79(135)52-115-78(134)51-116-82(138)61(102)45-57-31-33-59(131)34-32-57)94(150)122-67(29-19-41-113-99(109)110)95(151)130-42-20-30-75(130)93(149)121-64(26-14-16-38-101)85(141)124-69(43-53(2)3)87(143)119-63(25-13-15-37-100)84(140)126-72(47-58-50-114-62-24-12-11-23-60(58)62)90(146)128-74(49-80(136)137)92(148)127-73(48-77(104)133)91(147)123-68(96(152)153)35-36-76(103)132/h8-12,21-24,31-34,50,53-55,61,63-75,81,114,131H,7,13-20,25-30,35-49,51-52,100-102H2,1-6H3,(H2,103,132)(H2,104,133)(H,115,134)(H,116,138)(H,117,135)(H,118,139)(H,119,143)(H,120,144)(H,121,149)(H,122,150)(H,123,147)(H,124,141)(H,125,145)(H,126,140)(H,127,148)(H,128,146)(H,129,142)(H,136,137)(H,152,153)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t55-,61-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.231 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010702

(CHEMBL411557 | DYNORPHIN(1-17)-OH | Dynorphin A (1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r,wU:38.46,19.26,4.4,2.2,88.90,128.132,144.148,70.71,105.107,wD:57.58,30.34,8.15,84.87,97.99,114.116,136.140,(27.24,-21.93,;25.83,-21.28,;25.7,-19.76,;26.97,-18.87,;24.28,-19.11,;24.14,-17.56,;25.39,-16.68,;26.8,-17.34,;25.25,-15.13,;23.87,-14.49,;23.73,-12.97,;22.32,-12.32,;22.18,-10.79,;20.78,-10.14,;19.51,-11.03,;20.63,-8.6,;26.5,-14.25,;26.41,-12.7,;25.05,-12.02,;27.68,-11.87,;29.07,-12.56,;30.37,-11.71,;31.74,-12.4,;33.01,-11.53,;34.37,-12.24,;34.46,-13.77,;35.67,-11.39,;27.59,-10.31,;28.87,-9.48,;30.26,-10.18,;28.78,-7.92,;27.42,-7.24,;26.12,-8.09,;24.76,-7.41,;26.21,-9.64,;30.05,-7.09,;29.97,-5.53,;28.6,-4.85,;31.24,-4.7,;32.63,-5.4,;32.72,-6.92,;31.45,-7.77,;31.55,-9.31,;32.93,-9.99,;34.21,-9.14,;34.11,-7.6,;31.15,-3.14,;32.43,-2.31,;33.81,-3.01,;32.34,-.76,;33.61,.08,;33.52,1.63,;32.16,2.32,;34.8,2.47,;34.71,4.01,;36,4.86,;37.37,4.17,;35.91,6.4,;37.19,7.24,;34.53,7.1,;33.23,6.24,;33.34,4.69,;32.06,3.84,;30.68,4.52,;29.39,3.71,;30.58,6.06,;31.86,6.91,;23.04,-19.99,;21.65,-19.34,;23.18,-21.52,;21.92,-22.42,;22.06,-23.97,;20.82,-24.84,;19.43,-24.2,;18.16,-25.1,;16.78,-24.46,;16.64,-22.94,;15.51,-25.35,;20.12,-21.58,;18.85,-22.48,;19.94,-19.61,;21.09,-18.4,;20.31,-16.91,;18.66,-17.19,;18.69,-18.75,;17.33,-19.47,;17.28,-21.01,;16.02,-18.66,;14.66,-19.38,;14.61,-20.92,;13.25,-21.64,;13.2,-23.18,;11.84,-23.91,;11.79,-25.45,;13.36,-18.57,;13.41,-17.03,;12,-19.29,;10.69,-18.48,;10.74,-16.94,;9.44,-16.12,;9.49,-14.58,;8.08,-16.85,;9.33,-19.2,;9.28,-20.74,;8.03,-18.39,;6.67,-19.11,;6.61,-20.65,;5.26,-21.37,;5.2,-22.91,;3.85,-23.64,;3.79,-25.18,;5.36,-18.3,;5.41,-16.76,;4,-19.02,;2.69,-18.21,;2.75,-16.67,;1.44,-15.85,;.01,-16.43,;-.98,-15.25,;-.16,-13.95,;-.59,-12.47,;.48,-11.36,;1.97,-11.73,;2.4,-13.21,;1.33,-14.32,;1.34,-18.93,;1.28,-20.47,;.03,-18.12,;-1.33,-18.84,;-1.38,-20.38,;-2.74,-21.1,;-4.05,-20.29,;-2.79,-22.64,;-2.64,-18.03,;-2.59,-16.49,;-4,-18.75,;-5.3,-17.94,;-5.25,-16.4,;-6.56,-15.58,;-7.92,-16.31,;-6.51,-14.04,;-6.66,-18.66,;-6.71,-20.2,;-7.97,-17.85,;-9.33,-18.57,;-9.38,-20.11,;-10.74,-20.83,;-10.79,-22.37,;-9.48,-23.19,;-12.15,-23.1,;-10.63,-17.76,;-11.99,-18.48,;-10.58,-16.22,)| Show InChI InChI=1S/C99H155N31O23/c1-7-55(6)81(129-86(142)66(28-18-40-112-98(107)108)118-83(139)65(27-17-39-111-97(105)106)120-88(144)70(44-54(4)5)125-89(145)71(46-56-21-9-8-10-22-56)117-79(135)52-115-78(134)51-116-82(138)61(102)45-57-31-33-59(131)34-32-57)94(150)122-67(29-19-41-113-99(109)110)95(151)130-42-20-30-75(130)93(149)121-64(26-14-16-38-101)85(141)124-69(43-53(2)3)87(143)119-63(25-13-15-37-100)84(140)126-72(47-58-50-114-62-24-12-11-23-60(58)62)90(146)128-74(49-80(136)137)92(148)127-73(48-77(104)133)91(147)123-68(96(152)153)35-36-76(103)132/h8-12,21-24,31-34,50,53-55,61,63-75,81,114,131H,7,13-20,25-30,35-49,51-52,100-102H2,1-6H3,(H2,103,132)(H2,104,133)(H,115,134)(H,116,138)(H,117,135)(H,118,139)(H,119,143)(H,120,144)(H,121,149)(H,122,150)(H,123,147)(H,124,141)(H,125,145)(H,126,140)(H,127,148)(H,128,146)(H,129,142)(H,136,137)(H,152,153)(H4,105,106,111)(H4,107,108,112)(H4,109,110,113)/t55-,61-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.231 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010705

(CHEMBL385759 | H-Tyr-Gly-Gly-Phe-Cys-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C57H89N21O12S2/c1-3-31(2)45-53(89)74-38(15-9-23-68-57(64)65)54(90)78-24-10-16-42(78)52(88)75-40(46(59)82)29-91-92-30-41(51(87)73-36(13-7-21-66-55(60)61)48(84)72-37(49(85)77-45)14-8-22-67-56(62)63)76-50(86)39(26-32-11-5-4-6-12-32)71-44(81)28-69-43(80)27-70-47(83)35(58)25-33-17-19-34(79)20-18-33/h4-6,11-12,17-20,31,35-42,45,79H,3,7-10,13-16,21-30,58H2,1-2H3,(H2,59,82)(H,69,80)(H,70,83)(H,71,81)(H,72,84)(H,73,87)(H,74,89)(H,75,88)(H,76,86)(H,77,85)(H4,60,61,66)(H4,62,63,67)(H4,64,65,68)/t31-,35-,36-,37-,38-,39-,40-,41-,42-,45-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.285 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50010701

(CHEMBL410616 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-D-Cys...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O Show InChI InChI=1S/C69H112N24O14S2/c1-38(2)30-48(90-63(104)50(33-40-14-6-5-7-15-40)84-55(96)35-82-54(95)34-83-57(98)43(71)32-41-21-23-42(94)24-22-41)61(102)86-45(17-10-26-79-67(73)74)58(99)85-46(18-11-27-80-68(75)76)60(101)92-52-37-109-108-36-51(56(72)97)91-62(103)49(31-39(3)4)89-59(100)44(16-8-9-25-70)87-65(106)53-20-13-29-93(53)66(107)47(88-64(52)105)19-12-28-81-69(77)78/h5-7,14-15,21-24,38-39,43-53,94H,8-13,16-20,25-37,70-71H2,1-4H3,(H2,72,97)(H,82,95)(H,83,98)(H,84,96)(H,85,99)(H,86,102)(H,87,106)(H,88,105)(H,89,100)(H,90,104)(H,91,103)(H,92,101)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,44-,45-,46-,47-,48-,49-,50-,51+,52+,53-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.362 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 in guinea pig brain homogenate using [3H]- PL-17 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50040125

(CHEMBL438274 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-c(Cys-A...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@@H](NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O Show InChI InChI=1S/C69H111N23O15S2/c1-38(2)30-48(89-62(102)50(33-40-14-6-5-7-15-40)83-55(95)35-81-54(94)34-82-56(96)43(71)32-41-21-23-42(93)24-22-41)60(100)85-45(17-10-26-78-67(72)73)57(97)84-46(18-11-27-79-68(74)75)59(99)90-51-36-108-109-37-52(66(106)107)91-61(101)49(31-39(3)4)88-58(98)44(16-8-9-25-70)86-64(104)53-20-13-29-92(53)65(105)47(87-63(51)103)19-12-28-80-69(76)77/h5-7,14-15,21-24,38-39,43-53,93H,8-13,16-20,25-37,70-71H2,1-4H3,(H,81,94)(H,82,96)(H,83,95)(H,84,97)(H,85,100)(H,86,104)(H,87,103)(H,88,98)(H,89,102)(H,90,99)(H,91,101)(H,106,107)(H4,72,73,78)(H4,74,75,79)(H4,76,77,80)/t43-,44-,45-,46-,47-,48-,49+,50-,51-,52+,53+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PL-17 binding to mu opioid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50010700
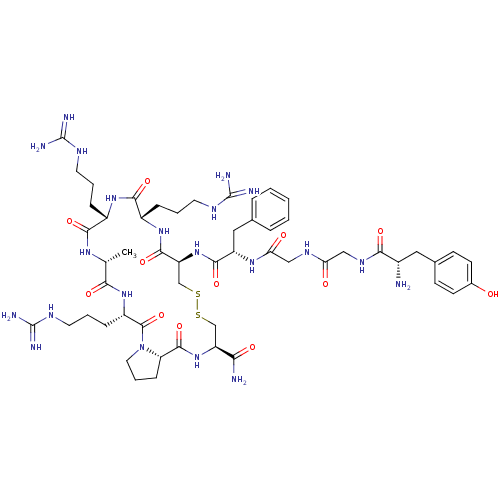
(CHEMBL265824 | H-Tyr-Gly-Gly-Phe-Cys-Arg-Arg-D-Ala...)Show SMILES C[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C54H83N21O12S2/c1-29-44(80)72-36(13-7-21-65-54(61)62)51(87)75-22-8-14-40(75)50(86)73-38(43(56)79)27-88-89-28-39(49(85)71-35(12-6-20-64-53(59)60)47(83)70-34(46(82)68-29)11-5-19-63-52(57)58)74-48(84)37(24-30-9-3-2-4-10-30)69-42(78)26-66-41(77)25-67-45(81)33(55)23-31-15-17-32(76)18-16-31/h2-4,9-10,15-18,29,33-40,76H,5-8,11-14,19-28,55H2,1H3,(H2,56,79)(H,66,77)(H,67,81)(H,68,82)(H,69,78)(H,70,83)(H,71,85)(H,72,80)(H,73,86)(H,74,84)(H4,57,58,63)(H4,59,60,64)(H4,61,62,65)/t29-,33+,34+,35+,36+,37+,38+,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.391 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor kappa 1 in guinea pig brain homogenate using [3H]- U-69593 as radioligand |
J Med Chem 33: 1874-9 (1990)
BindingDB Entry DOI: 10.7270/Q2FB51XH |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Rattus norvegicus (Rat)) | BDBM309489
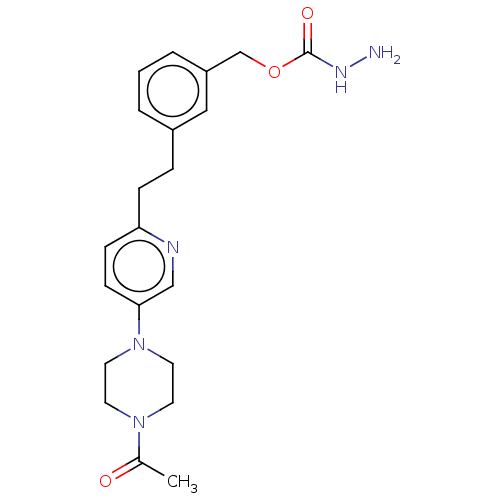
(US9603833, Example 111)Show SMILES CC(=O)N1CCN(CC1)c1ccc(CCc2cccc(COC(=O)NN)c2)nc1 Show InChI InChI=1S/C21H27N5O3/c1-16(27)25-9-11-26(12-10-25)20-8-7-19(23-14-20)6-5-17-3-2-4-18(13-17)15-29-21(28)24-22/h2-4,7-8,13-14H,5-6,9-12,15,22H2,1H3,(H,24,28) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd.
US Patent
| Assay Description
The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... |
US Patent US9603833 (2017)
BindingDB Entry DOI: 10.7270/Q2W37ZCW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040130
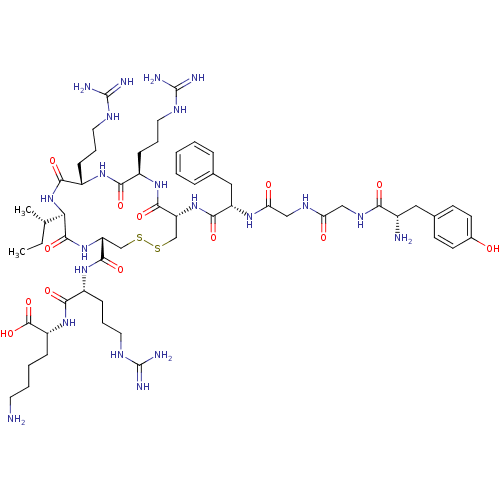
(CHEMBL415406 | Tyr-Gly-Gly-Phe-c(Cys-Arg-Arg-Ile-C...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](CSSC[C@@H](NC1=O)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H](CCCCN)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C58H93N21O13S2/c1-3-32(2)46-54(90)78-43(53(89)75-38(16-10-24-68-57(63)64)49(85)76-40(55(91)92)14-7-8-22-59)31-94-93-30-42(52(88)74-37(15-9-23-67-56(61)62)48(84)73-39(50(86)79-46)17-11-25-69-58(65)66)77-51(87)41(27-33-12-5-4-6-13-33)72-45(82)29-70-44(81)28-71-47(83)36(60)26-34-18-20-35(80)21-19-34/h4-6,12-13,18-21,32,36-43,46,80H,3,7-11,14-17,22-31,59-60H2,1-2H3,(H,70,81)(H,71,83)(H,72,82)(H,73,84)(H,74,88)(H,75,89)(H,76,85)(H,77,87)(H,78,90)(H,79,86)(H,91,92)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t32-,36-,37+,38+,39-,40+,41-,42+,43+,46+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50040126

(CHEMBL265241 | Tyr-Gly-Gly-Phe-leu-c(Cys-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C58H92N18O13S2/c1-5-33(4)47-55(87)71-38(16-11-23-65-57(61)62)49(81)74-44(54(86)72-40(56(88)89)15-9-10-22-59)31-91-90-30-43(53(85)70-39(50(82)76-47)17-12-24-66-58(63)64)75-51(83)41(25-32(2)3)73-52(84)42(27-34-13-7-6-8-14-34)69-46(79)29-67-45(78)28-68-48(80)37(60)26-35-18-20-36(77)21-19-35/h6-8,13-14,18-21,32-33,37-44,47,77H,5,9-12,15-17,22-31,59-60H2,1-4H3,(H,67,78)(H,68,80)(H,69,79)(H,70,85)(H,71,87)(H,72,86)(H,73,84)(H,74,81)(H,75,83)(H,76,82)(H,88,89)(H4,61,62,65)(H4,63,64,66)/t33-,37-,38-,39-,40-,41-,42-,43-,44-,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.475 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-DPDPE binding to delta opioid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd.
US Patent
| Assay Description
The compounds of the present invention obtained in the Production Examples were examined for the inhibitory effect on human monoamineoxydase enzymes ... |
US Patent US9603833 (2017)
BindingDB Entry DOI: 10.7270/Q2W37ZCW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Membrane primary amine oxidase
(Homo sapiens (Human)) | BDBM309489

(US9603833, Example 111)Show SMILES CC(=O)N1CCN(CC1)c1ccc(CCc2cccc(COC(=O)NN)c2)nc1 Show InChI InChI=1S/C21H27N5O3/c1-16(27)25-9-11-26(12-10-25)20-8-7-19(23-14-20)6-5-17-3-2-4-18(13-17)15-29-21(28)24-22/h2-4,7-8,13-14H,5-6,9-12,15,22H2,1H3,(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd.
US Patent
| Assay Description
The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... |
US Patent US9603833 (2017)
BindingDB Entry DOI: 10.7270/Q2W37ZCW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040124
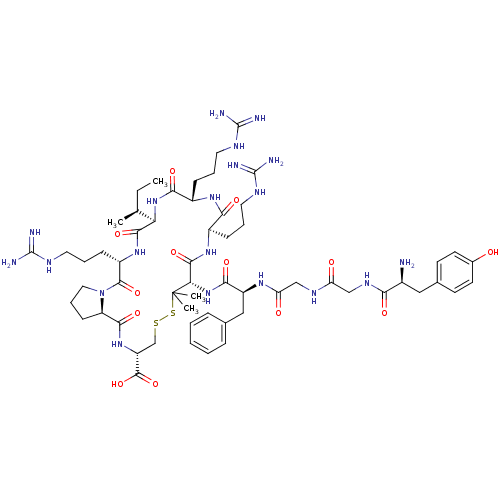
(CHEMBL414494 | Tyr-Gly-Gly-Phe-c((L-Pen)-Arg-Arg-I...)Show SMILES CC[C@H](C)[C@H]1NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](NC(=O)[C@H](Cc2ccccc2)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc2ccc(O)cc2)C(C)(C)SSC[C@@H](NC(=O)[C@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC1=O)C(O)=O Show InChI InChI=1S/C59H92N20O13S2/c1-5-32(2)45-52(88)75-39(17-11-25-69-58(65)66)54(90)79-26-12-18-42(79)51(87)76-41(55(91)92)31-93-94-59(3,4)46(53(89)74-37(15-9-23-67-56(61)62)48(84)73-38(49(85)77-45)16-10-24-68-57(63)64)78-50(86)40(28-33-13-7-6-8-14-33)72-44(82)30-70-43(81)29-71-47(83)36(60)27-34-19-21-35(80)22-20-34/h6-8,13-14,19-22,32,36-42,45-46,80H,5,9-12,15-18,23-31,60H2,1-4H3,(H,70,81)(H,71,83)(H,72,82)(H,73,84)(H,74,89)(H,75,88)(H,76,87)(H,77,85)(H,78,86)(H,91,92)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t32-,36-,37+,38-,39-,40-,41+,42+,45+,46-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.522 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
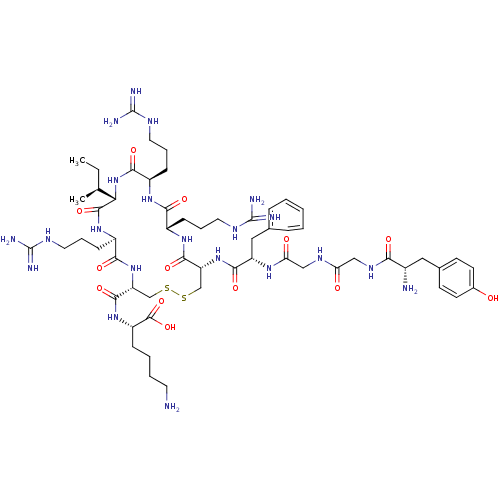
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50040133
(CHEMBL444626 | Tyr-Gly-Gly-Phe-c(Cys-Arg-Arg-Ile-A...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1 Show InChI InChI=1S/C58H93N21O13S2/c1-3-32(2)46-54(90)75-38(16-10-24-68-57(63)64)49(85)77-43(53(89)76-40(55(91)92)14-7-8-22-59)31-94-93-30-42(52(88)74-37(15-9-23-67-56(61)62)48(84)73-39(50(86)79-46)17-11-25-69-58(65)66)78-51(87)41(27-33-12-5-4-6-13-33)72-45(82)29-70-44(81)28-71-47(83)36(60)26-34-18-20-35(80)21-19-34/h4-6,12-13,18-21,32,36-43,46,80H,3,7-11,14-17,22-31,59-60H2,1-2H3,(H,70,81)(H,71,83)(H,72,82)(H,73,84)(H,74,88)(H,75,90)(H,76,89)(H,77,85)(H,78,87)(H,79,86)(H,91,92)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t32-,36-,37-,38+,39+,40-,41-,42+,43+,46-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.593 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-U-69,593 binding to kappa opoid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Rattus norvegicus (Rat)) | BDBM309497
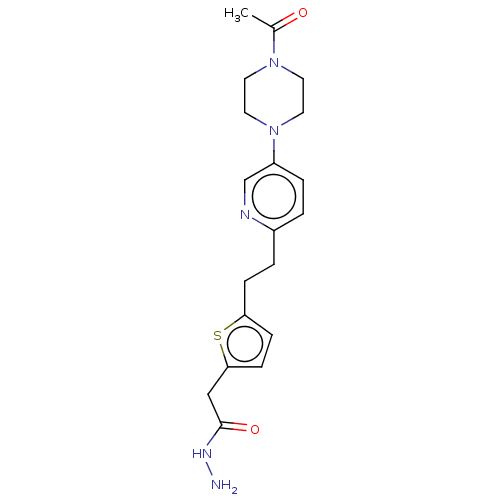
(US9603833, Example 120)Show SMILES CC(=O)N1CCN(CC1)c1ccc(CCc2ccc(CC(=O)NN)s2)nc1 |$;;;;;;;;;;;;;;;;;;;;;;HN;;;;$| Show InChI InChI=1S/C19H25N5O2S/c1-14(25)23-8-10-24(11-9-23)16-4-2-15(21-13-16)3-5-17-6-7-18(27-17)12-19(26)22-20/h2,4,6-7,13H,3,5,8-12,20H2,1H3,(H,22,26) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd.
US Patent
| Assay Description
The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... |
US Patent US9603833 (2017)
BindingDB Entry DOI: 10.7270/Q2W37ZCW |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50040131

(CHEMBL409793 | Tyr-Gly-Gly-Phe-Leu-Arg-Arg-c((D-Cy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]1CSSC[C@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](CCCNC(N)=N)NC1=O)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C69H112N24O15S2/c1-39(2)32-49(90-61(102)50(34-40-14-4-3-5-15-40)84-55(96)36-82-54(95)35-83-56(97)43(72)33-41-22-24-42(94)25-23-41)60(101)86-45(18-10-28-79-67(73)74)57(98)85-46(19-11-29-80-68(75)76)59(100)91-51-37-109-110-38-52(63(104)89-48(66(107)108)17-7-9-27-71)92-58(99)44(16-6-8-26-70)87-64(105)53-21-13-31-93(53)65(106)47(88-62(51)103)20-12-30-81-69(77)78/h3-5,14-15,22-25,39,43-53,94H,6-13,16-21,26-38,70-72H2,1-2H3,(H,82,95)(H,83,97)(H,84,96)(H,85,98)(H,86,101)(H,87,105)(H,88,103)(H,89,104)(H,90,102)(H,91,100)(H,92,99)(H,107,108)(H4,73,74,79)(H4,75,76,80)(H4,77,78,81)/t43-,44+,45-,46-,47+,48-,49-,50-,51+,52-,53-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.674 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-PL-17 binding to mu opioid receptor of guinea pig brain homogenate |
J Med Chem 36: 750-7 (1993)
BindingDB Entry DOI: 10.7270/Q2K936K7 |
More data for this
Ligand-Target Pair | |
Membrane primary amine oxidase
(Rattus norvegicus (Rat)) | BDBM309490
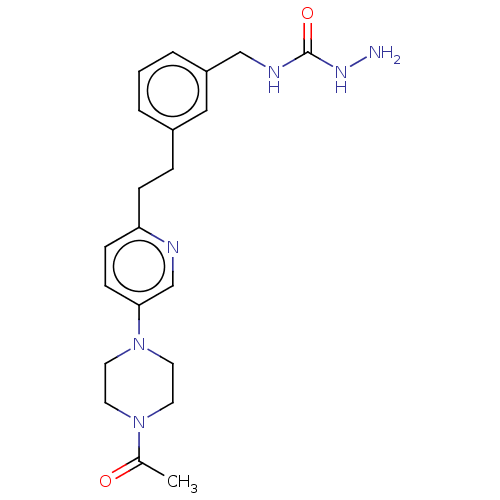
(US9603833, Example 112)Show SMILES CC(=O)N1CCN(CC1)c1ccc(CCc2cccc(CNC(=O)NN)c2)nc1 Show InChI InChI=1S/C21H28N6O2/c1-16(28)26-9-11-27(12-10-26)20-8-7-19(23-15-20)6-5-17-3-2-4-18(13-17)14-24-21(29)25-22/h2-4,7-8,13,15H,5-6,9-12,14,22H2,1H3,(H2,24,25,29) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
R-Tech Ueno, Ltd.
US Patent
| Assay Description
The compounds of the present invention obtained in Production Examples were examined for the inhibitory effect on human and rat VAP-1 enzyme (SSAO) b... |
US Patent US9603833 (2017)
BindingDB Entry DOI: 10.7270/Q2W37ZCW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































