 Found 202 hits with Last Name = 'keller' and Initial = 'pa'
Found 202 hits with Last Name = 'keller' and Initial = 'pa' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527096
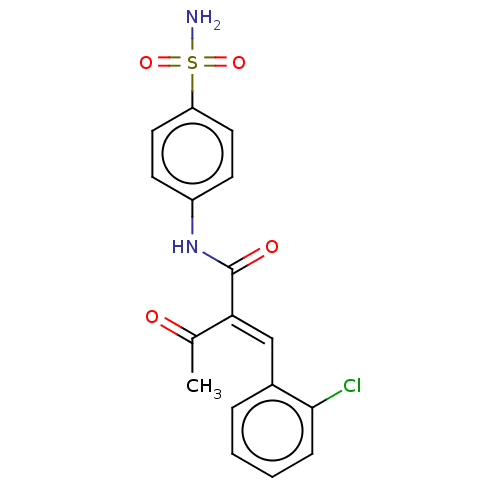
(CHEMBL4558452)Show SMILES CC(=O)C(=C/c1ccccc1Cl)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15ClN2O4S/c1-11(21)15(10-12-4-2-3-5-16(12)18)17(22)20-13-6-8-14(9-7-13)25(19,23)24/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527087

(CHEMBL4450169)Show SMILES COc1ccc(\C=C(/C(C)=O)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C18H18N2O5S/c1-12(21)17(11-13-3-7-15(25-2)8-4-13)18(22)20-14-5-9-16(10-6-14)26(19,23)24/h3-11H,1-2H3,(H,20,22)(H2,19,23,24)/b17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615173

(CHEMBL5265782) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527086
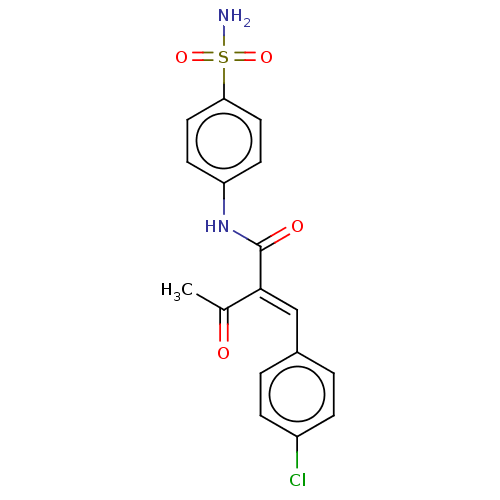
(CHEMBL4458973)Show SMILES CC(=O)C(=C/c1ccc(Cl)cc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15ClN2O4S/c1-11(21)16(10-12-2-4-13(18)5-3-12)17(22)20-14-6-8-15(9-7-14)25(19,23)24/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50158804
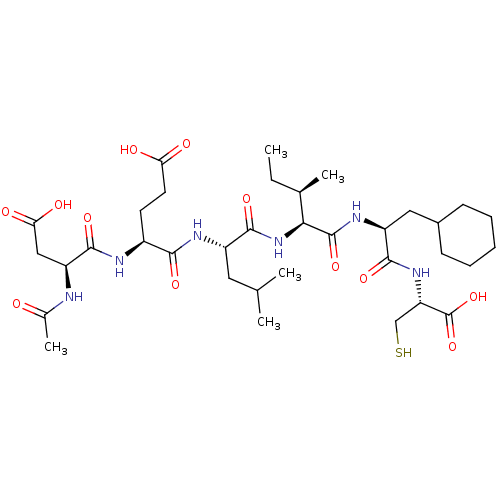
(AcAsp-D-Gla-Leu-Ile-Cha-Cys | CHEMBL179084)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C35H58N6O12S/c1-6-19(4)29(34(51)39-24(15-21-10-8-7-9-11-21)31(48)40-26(17-54)35(52)53)41-33(50)23(14-18(2)3)38-30(47)22(12-13-27(43)44)37-32(49)25(16-28(45)46)36-20(5)42/h18-19,21-26,29,54H,6-17H2,1-5H3,(H,36,42)(H,37,49)(H,38,47)(H,39,51)(H,40,48)(H,41,50)(H,43,44)(H,45,46)(H,52,53)/t19-,22+,23+,24+,25+,26+,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibitory constant against hepatitis C virus NS3 protease |
J Med Chem 48: 1-20 (2005)
Article DOI: 10.1021/jm0400101
BindingDB Entry DOI: 10.7270/Q2XP75Q1 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615175
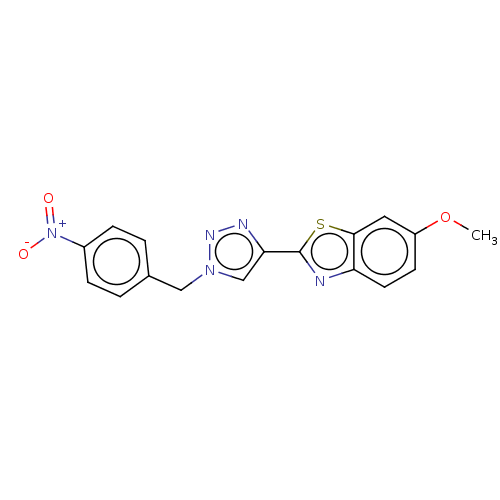
(CHEMBL5285051)Show SMILES COc1ccc2nc(sc2c1)-c1cn(Cc2ccc(cc2)[N+]([O-])=O)nn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | >1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615166

(CHEMBL5287804) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | >1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50054245
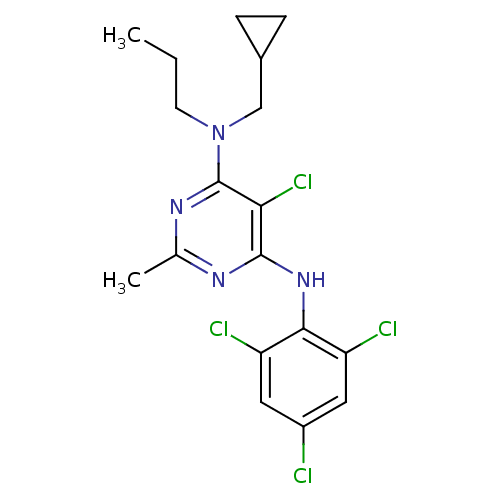
(5-Chloro-N-cyclopropylmethyl-2-methyl-N-propyl-N''...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1Cl Show InChI InChI=1S/C18H20Cl4N4/c1-3-6-26(9-11-4-5-11)18-15(22)17(23-10(2)24-18)25-16-13(20)7-12(19)8-14(16)21/h7-8,11H,3-6,9H2,1-2H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CRH binding to human Corticotropin releasing factor receptor 1. |
J Med Chem 42: 2351-7 (1999)
Article DOI: 10.1021/jm9900117
BindingDB Entry DOI: 10.7270/Q2WS8SF0 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50054252
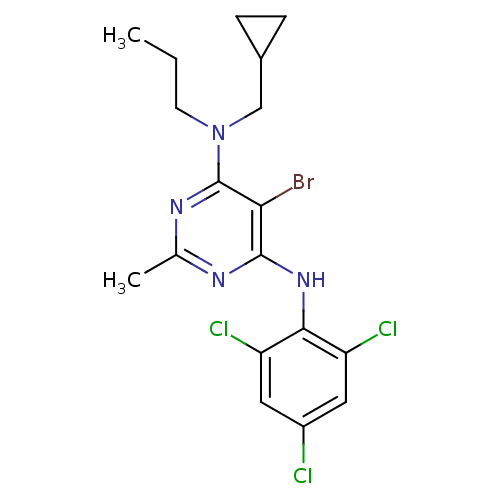
(5-Bromo-N-cyclopropylmethyl-2-methyl-N-propyl-N'-(...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1Br Show InChI InChI=1S/C18H20BrCl3N4/c1-3-6-26(9-11-4-5-11)18-15(19)17(23-10(2)24-18)25-16-13(21)7-12(20)8-14(16)22/h7-8,11H,3-6,9H2,1-2H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]-CRH binding to human Corticotropin releasing factor receptor 1 |
J Med Chem 42: 2351-7 (1999)
Article DOI: 10.1021/jm9900117
BindingDB Entry DOI: 10.7270/Q2WS8SF0 |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50054250
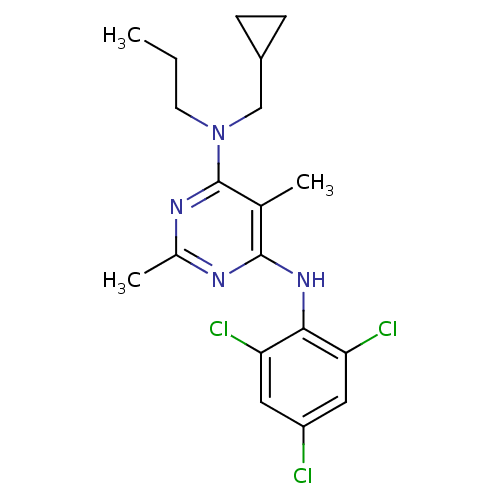
(CHEMBL306482 | N-Cyclopropylmethyl-2,5-dimethyl-N-...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1C Show InChI InChI=1S/C19H23Cl3N4/c1-4-7-26(10-13-5-6-13)19-11(2)18(23-12(3)24-19)25-17-15(21)8-14(20)9-16(17)22/h8-9,13H,4-7,10H2,1-3H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CRH binding to human Corticotropin releasing factor receptor 1. |
J Med Chem 42: 2351-7 (1999)
Article DOI: 10.1021/jm9900117
BindingDB Entry DOI: 10.7270/Q2WS8SF0 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615163
(CHEMBL5272792) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50054248
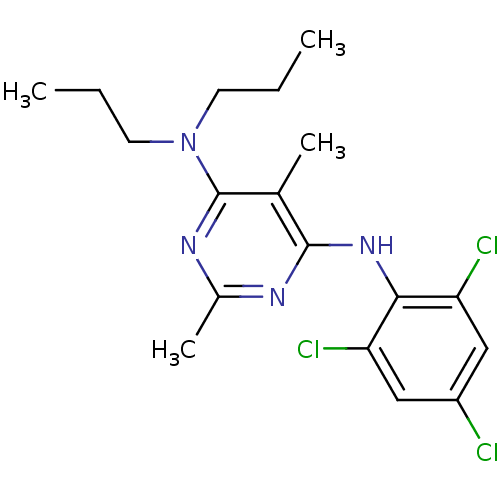
(2,5-Dimethyl-N,N-dipropyl-N'-(2,4,6-trichloro-phen...)Show InChI InChI=1S/C18H23Cl3N4/c1-5-7-25(8-6-2)18-11(3)17(22-12(4)23-18)24-16-14(20)9-13(19)10-15(16)21/h9-10H,5-8H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CRH binding to human Corticotropin releasing factor receptor 1. |
J Med Chem 42: 2351-7 (1999)
Article DOI: 10.1021/jm9900117
BindingDB Entry DOI: 10.7270/Q2WS8SF0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527096

(CHEMBL4558452)Show SMILES CC(=O)C(=C/c1ccccc1Cl)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15ClN2O4S/c1-11(21)15(10-12-4-2-3-5-16(12)18)17(22)20-13-6-8-14(9-7-13)25(19,23)24/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b15-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527077
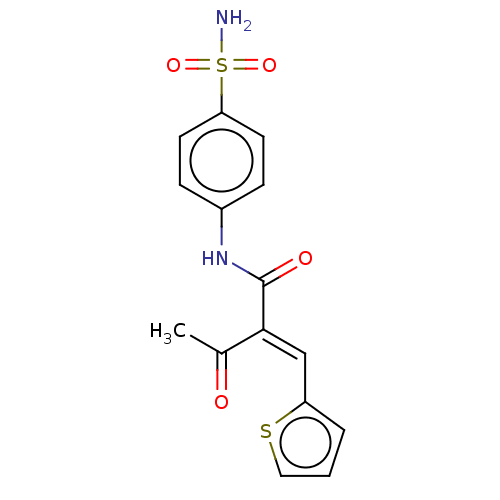
(CHEMBL4455089)Show SMILES CC(=O)C(=C/c1cccs1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H14N2O4S2/c1-10(18)14(9-12-3-2-8-22-12)15(19)17-11-4-6-13(7-5-11)23(16,20)21/h2-9H,1H3,(H,17,19)(H2,16,20,21)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10885

((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...)Show SMILES CCN[C@H]1CN(CCCOC)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C12H21N3O5S3/c1-3-14-10-8-15(5-4-6-20-2)23(18,19)12-9(10)7-11(21-12)22(13,16)17/h7,10,14H,3-6,8H2,1-2H3,(H2,13,16,17)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527094
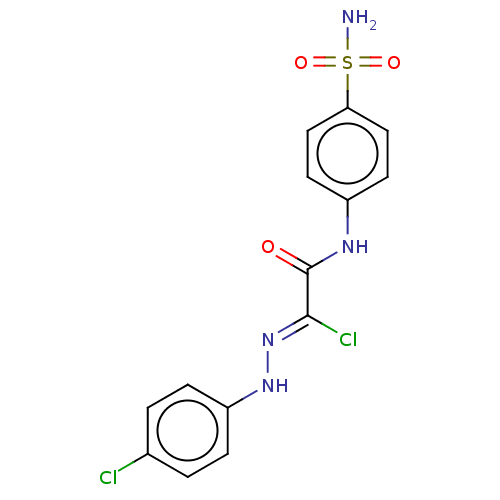
(CHEMBL4577572)Show SMILES NS(=O)(=O)c1ccc(NC(=O)C(\Cl)=N\Nc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C14H12Cl2N4O3S/c15-9-1-3-11(4-2-9)19-20-13(16)14(21)18-10-5-7-12(8-6-10)24(17,22)23/h1-8,19H,(H,18,21)(H2,17,22,23)/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527087

(CHEMBL4450169)Show SMILES COc1ccc(\C=C(/C(C)=O)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C18H18N2O5S/c1-12(21)17(11-13-3-7-15(25-2)8-4-13)18(22)20-14-5-9-16(10-6-14)26(19,23)24/h3-11H,1-2H3,(H,20,22)(H2,19,23,24)/b17-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50054253
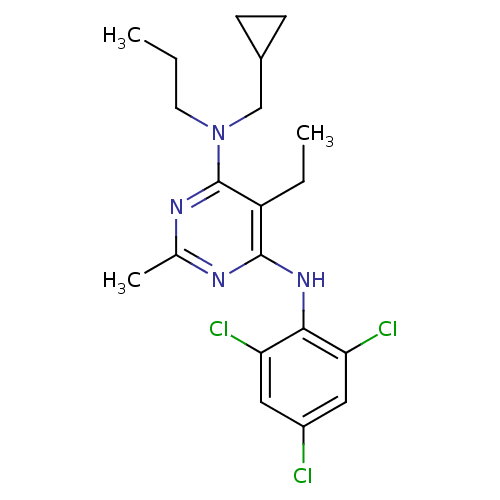
(CHEMBL77716 | N-Cyclopropylmethyl-5-ethyl-2-methyl...)Show SMILES CCCN(CC1CC1)c1nc(C)nc(Nc2c(Cl)cc(Cl)cc2Cl)c1CC Show InChI InChI=1S/C20H25Cl3N4/c1-4-8-27(11-13-6-7-13)20-15(5-2)19(24-12(3)25-20)26-18-16(22)9-14(21)10-17(18)23/h9-10,13H,4-8,11H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-CRH binding to human Corticotropin releasing factor receptor 1. |
J Med Chem 42: 2351-7 (1999)
Article DOI: 10.1021/jm9900117
BindingDB Entry DOI: 10.7270/Q2WS8SF0 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615179

(CHEMBL5270129) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527088
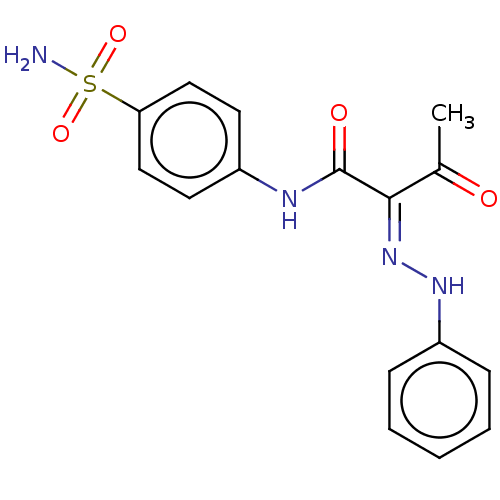
(CHEMBL4525908)Show SMILES CC(=O)C(=N/Nc1ccccc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H16N4O4S/c1-11(21)15(20-19-13-5-3-2-4-6-13)16(22)18-12-7-9-14(10-8-12)25(17,23)24/h2-10,19H,1H3,(H,18,22)(H2,17,23,24)/b20-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527086

(CHEMBL4458973)Show SMILES CC(=O)C(=C/c1ccc(Cl)cc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15ClN2O4S/c1-11(21)16(10-12-2-4-13(18)5-3-12)17(22)20-14-6-8-15(9-7-14)25(19,23)24/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527082
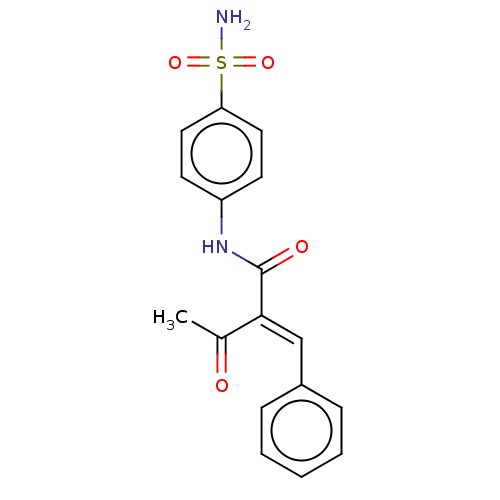
(CHEMBL4447914)Show SMILES CC(=O)C(=C/c1ccccc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H16N2O4S/c1-12(20)16(11-13-5-3-2-4-6-13)17(21)19-14-7-9-15(10-8-14)24(18,22)23/h2-11H,1H3,(H,19,21)(H2,18,22,23)/b16-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527092
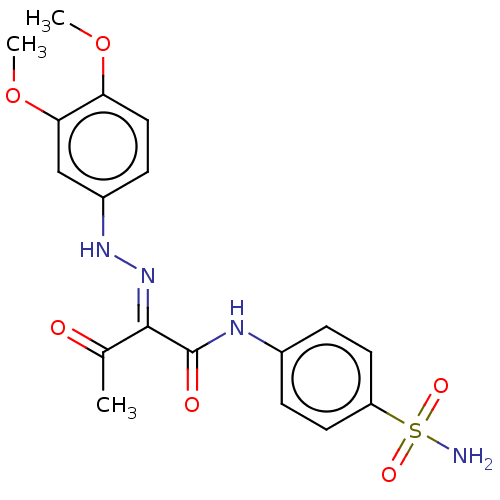
(CHEMBL4593779)Show SMILES COc1ccc(N\N=C(/C(C)=O)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1OC Show InChI InChI=1S/C18H20N4O6S/c1-11(23)17(22-21-13-6-9-15(27-2)16(10-13)28-3)18(24)20-12-4-7-14(8-5-12)29(19,25)26/h4-10,21H,1-3H3,(H,20,24)(H2,19,25,26)/b22-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527092

(CHEMBL4593779)Show SMILES COc1ccc(N\N=C(/C(C)=O)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1OC Show InChI InChI=1S/C18H20N4O6S/c1-11(23)17(22-21-13-6-9-15(27-2)16(10-13)28-3)18(24)20-12-4-7-14(8-5-12)29(19,25)26/h4-10,21H,1-3H3,(H,20,24)(H2,19,25,26)/b22-17+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527090
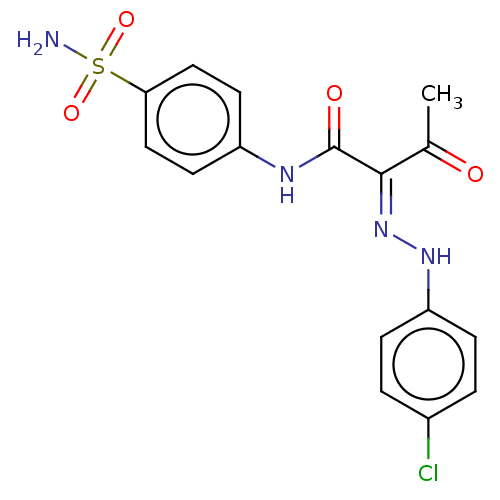
(CHEMBL4483065)Show SMILES CC(=O)C(=N/Nc1ccc(Cl)cc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H15ClN4O4S/c1-10(22)15(21-20-13-4-2-11(17)3-5-13)16(23)19-12-6-8-14(9-7-12)26(18,24)25/h2-9,20H,1H3,(H,19,23)(H2,18,24,25)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527077

(CHEMBL4455089)Show SMILES CC(=O)C(=C/c1cccs1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H14N2O4S2/c1-10(18)14(9-12-3-2-8-22-12)15(19)17-11-4-6-13(7-5-11)23(16,20)21/h2-9H,1H3,(H,17,19)(H2,16,20,21)/b14-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527084

(CHEMBL4451455)Show SMILES CC(=O)C(=C/c1ccc(F)cc1Cl)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClFN2O4S/c1-10(22)15(8-11-2-3-12(19)9-16(11)18)17(23)21-13-4-6-14(7-5-13)26(20,24)25/h2-9H,1H3,(H,21,23)(H2,20,24,25)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527094

(CHEMBL4577572)Show SMILES NS(=O)(=O)c1ccc(NC(=O)C(\Cl)=N\Nc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C14H12Cl2N4O3S/c15-9-1-3-11(4-2-9)19-20-13(16)14(21)18-10-5-7-12(8-6-10)24(17,22)23/h1-8,19H,(H,18,21)(H2,17,22,23)/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615182
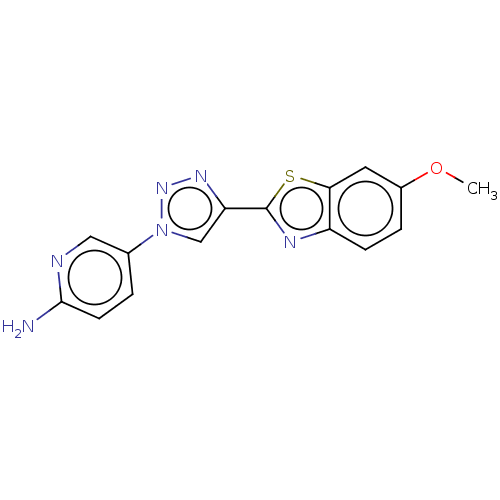
(CHEMBL5265987) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527083

(CHEMBL4557855)Show SMILES CC(=O)C(=C/c1cccc(c1)[N+]([O-])=O)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15N3O6S/c1-11(21)16(10-12-3-2-4-14(9-12)20(23)24)17(22)19-13-5-7-15(8-6-13)27(18,25)26/h2-10H,1H3,(H,19,22)(H2,18,25,26)/b16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50527096

(CHEMBL4558452)Show SMILES CC(=O)C(=C/c1ccccc1Cl)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H15ClN2O4S/c1-11(21)15(10-12-4-2-3-5-16(12)18)17(22)20-13-6-8-14(9-7-13)25(19,23)24/h2-10H,1H3,(H,20,22)(H2,19,23,24)/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 1 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615180
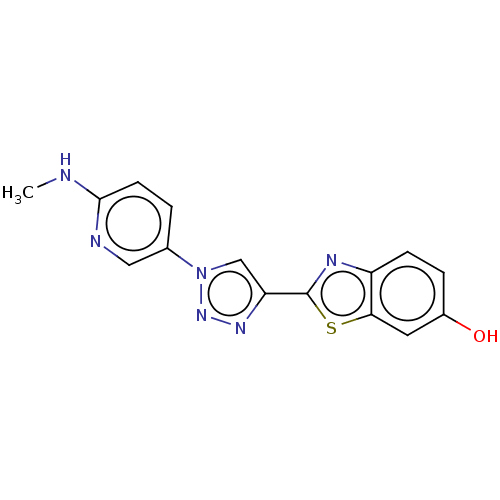
(CHEMBL5277173) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527089
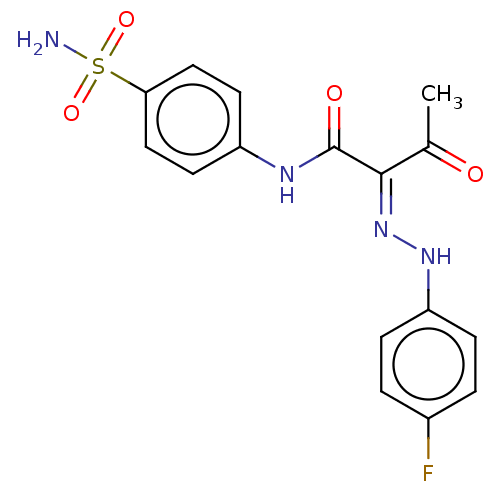
(CHEMBL4590738)Show SMILES CC(=O)C(=N/Nc1ccc(F)cc1)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H15FN4O4S/c1-10(22)15(21-20-13-4-2-11(17)3-5-13)16(23)19-12-6-8-14(9-7-12)26(18,24)25/h2-9,20H,1H3,(H,19,23)(H2,18,24,25)/b21-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10884

((2S,4S)-4-(ethylamino)-2-methyl-1,1-dioxo-2H,3H,4H...)Show SMILES CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O |r| Show InChI InChI=1S/C10H16N2O4S3/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16)/t6-,8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus) | BDBM50110121
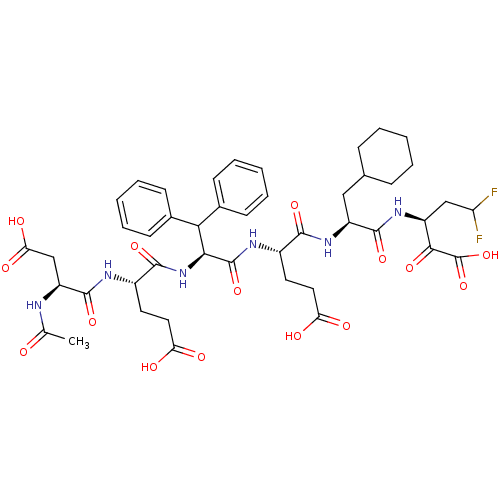
(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O Show InChI InChI=1S/C45H56F2N6O15/c1-24(54)48-32(23-36(59)60)43(65)49-29(18-20-35(57)58)41(63)53-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)44(66)50-28(17-19-34(55)56)40(62)52-31(21-25-11-5-2-6-12-25)42(64)51-30(22-33(46)47)39(61)45(67)68/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,48,54)(H,49,65)(H,50,66)(H,51,64)(H,52,62)(H,53,63)(H,55,56)(H,57,58)(H,59,60)(H,67,68)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibitory constant against hepatitis C virus NS3 protease |
J Med Chem 48: 1-20 (2005)
Article DOI: 10.1021/jm0400101
BindingDB Entry DOI: 10.7270/Q2XP75Q1 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615164
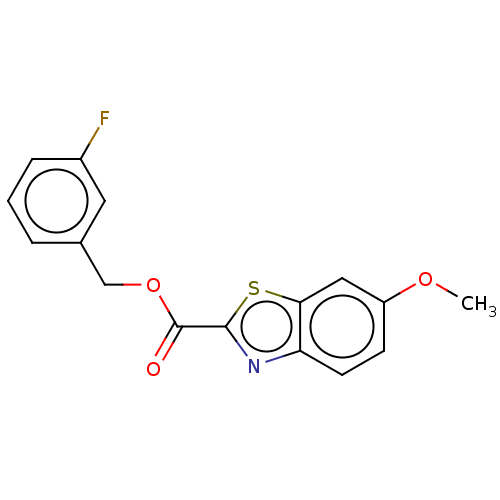
(CHEMBL5266253) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615168
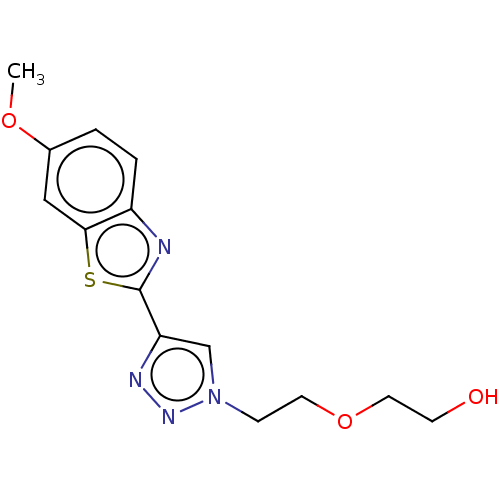
(CHEMBL5273005) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50527087

(CHEMBL4450169)Show SMILES COc1ccc(\C=C(/C(C)=O)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C18H18N2O5S/c1-12(21)17(11-13-3-7-15(25-2)8-4-13)18(22)20-14-5-9-16(10-6-14)26(19,23)24/h3-11H,1-2H3,(H,20,22)(H2,19,23,24)/b17-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 1 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527080
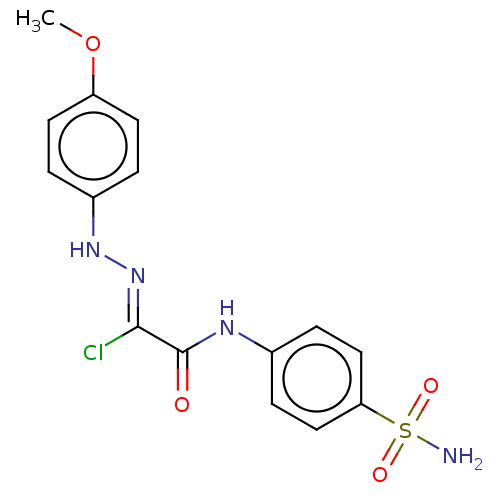
(CHEMBL4460385)Show SMILES COc1ccc(N\N=C(/Cl)C(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H15ClN4O4S/c1-24-12-6-2-11(3-7-12)19-20-14(16)15(21)18-10-4-8-13(9-5-10)25(17,22)23/h2-9,19H,1H3,(H,18,21)(H2,17,22,23)/b20-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50158796
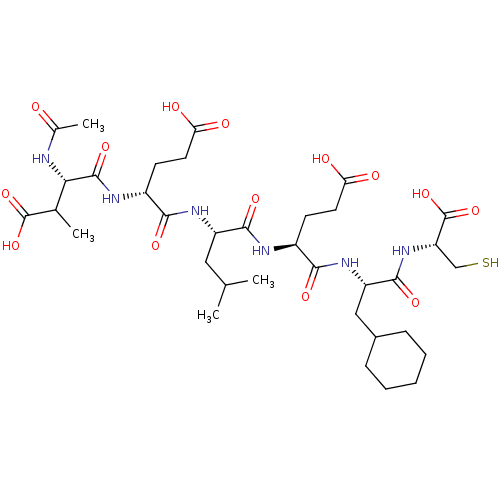
(AcAsp-D-Glu-Leu-Glu-Cha-Cys | CHEMBL360025)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@@H](NC(C)=O)C(C)C(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C35H56N6O14S/c1-17(2)14-23(39-30(48)22(11-13-27(45)46)38-33(51)28(36-19(4)42)18(3)34(52)53)31(49)37-21(10-12-26(43)44)29(47)40-24(15-20-8-6-5-7-9-20)32(50)41-25(16-56)35(54)55/h17-18,20-25,28,56H,5-16H2,1-4H3,(H,36,42)(H,37,49)(H,38,51)(H,39,48)(H,40,47)(H,41,50)(H,43,44)(H,45,46)(H,52,53)(H,54,55)/t18?,21-,22+,23-,24-,25-,28-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibitory constant against hepatitis C virus NS3 protease |
J Med Chem 48: 1-20 (2005)
Article DOI: 10.1021/jm0400101
BindingDB Entry DOI: 10.7270/Q2XP75Q1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 7
(Homo sapiens (Human)) | BDBM50527084

(CHEMBL4451455)Show SMILES CC(=O)C(=C/c1ccc(F)cc1Cl)\C(=O)Nc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C17H14ClFN2O4S/c1-10(22)15(8-11-2-3-12(19)9-16(11)18)17(23)21-13-4-6-14(7-5-13)26(20,24)25/h2-9H,1H3,(H,21,23)(H2,20,24,25)/b15-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 7 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615174
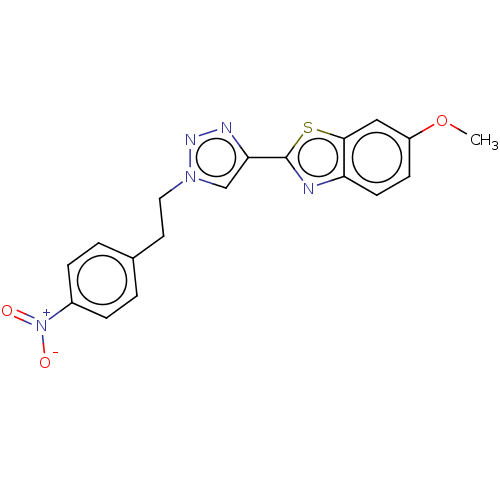
(CHEMBL5283139)Show SMILES COc1ccc2nc(sc2c1)-c1cn(CCc2ccc(cc2)[N+]([O-])=O)nn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615171
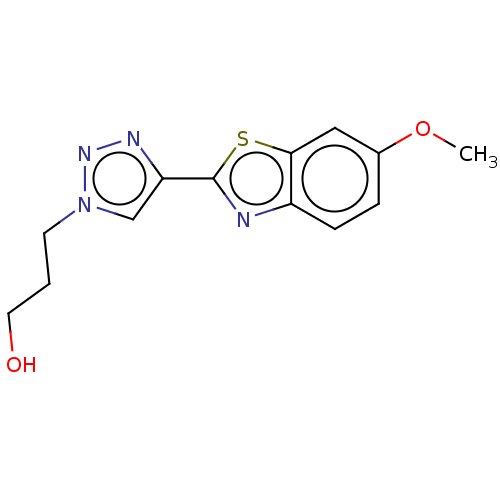
(CHEMBL5273003) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50527093
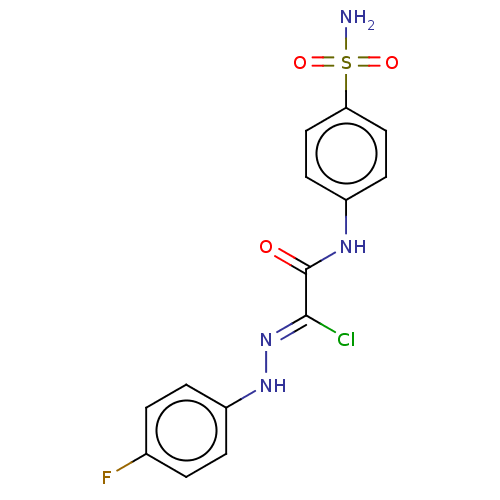
(CHEMBL4520993)Show SMILES NS(=O)(=O)c1ccc(NC(=O)C(\Cl)=N\Nc2ccc(F)cc2)cc1 Show InChI InChI=1S/C14H12ClFN4O3S/c15-13(20-19-11-3-1-9(16)2-4-11)14(21)18-10-5-7-12(8-6-10)24(17,22)23/h1-8,19H,(H,18,21)(H2,17,22,23)/b20-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 2 incubated for 6 hrs prior to testing measured for 10 to 100 secs by phenol red-based stopped-flo... |
J Med Chem 63: 3317-3326 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02090
BindingDB Entry DOI: 10.7270/Q25Q50JG |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615181
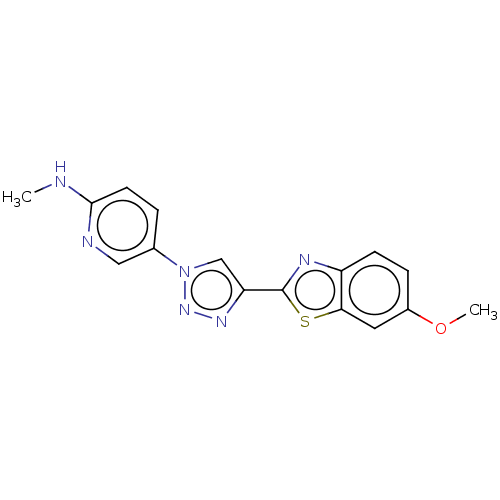
(CHEMBL5276358) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM50084685

(4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CS)C(O)=O Show InChI InChI=1S/C43H56N6O14S/c1-24(50)44-31(22-35(55)56)41(60)45-29(18-20-34(53)54)39(58)49-37(36(26-13-7-3-8-14-26)27-15-9-4-10-16-27)42(61)46-28(17-19-33(51)52)38(57)47-30(21-25-11-5-2-6-12-25)40(59)48-32(23-64)43(62)63/h3-4,7-10,13-16,25,28-32,36-37,64H,2,5-6,11-12,17-23H2,1H3,(H,44,50)(H,45,60)(H,46,61)(H,47,57)(H,48,59)(H,49,58)(H,51,52)(H,53,54)(H,55,56)(H,62,63)/t28-,29-,30-,31-,32-,37-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong
Curated by ChEMBL
| Assay Description
Inhibitory constant against hepatitis C virus NS3 protease |
J Med Chem 48: 1-20 (2005)
Article DOI: 10.1021/jm0400101
BindingDB Entry DOI: 10.7270/Q2XP75Q1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data