

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019296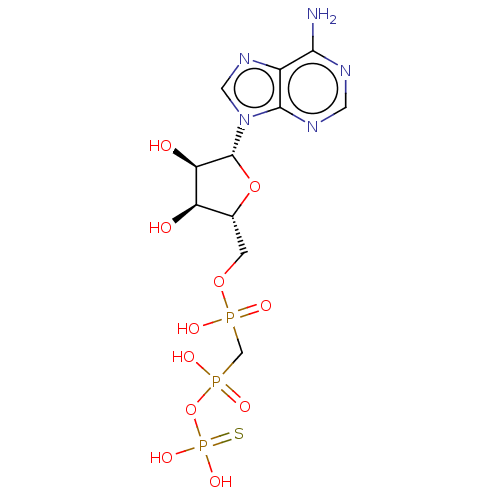 (CHEMBL3289396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019294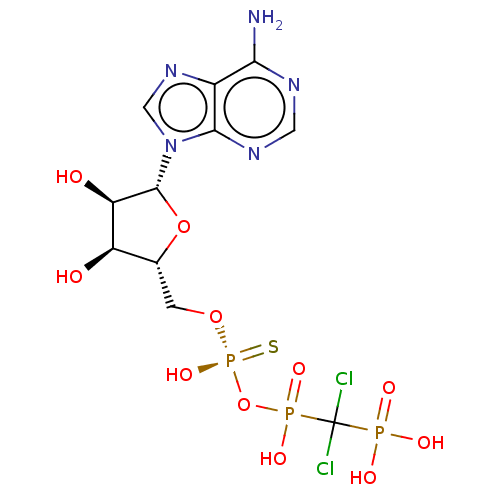 (CHEMBL3289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 685 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292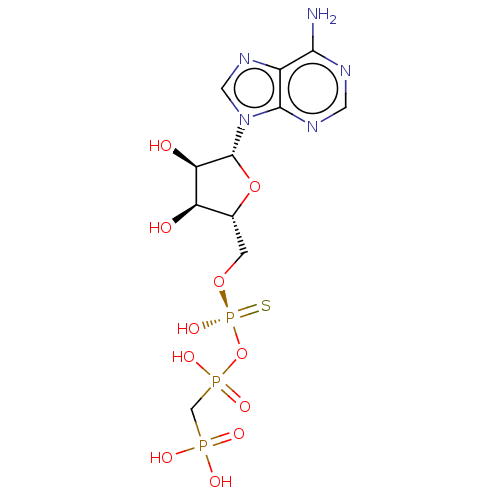 (CHEMBL3289393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291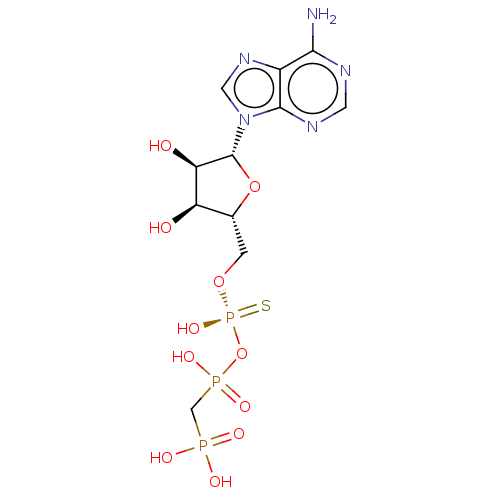 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate assessed as dissociation constant for enzyme-inhibitor-substrate complex after 20 mins... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019296 (CHEMBL3289396) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019294 (CHEMBL3289394) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019291 (CHEMBL3289392) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019292 (CHEMBL3289393) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50019295 (CHEMBL3289395) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Mixed type inhibition of human NPP1 using pNP-TMP as substrate after 20 mins by Dixon and Cornish-Bowden method | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50366480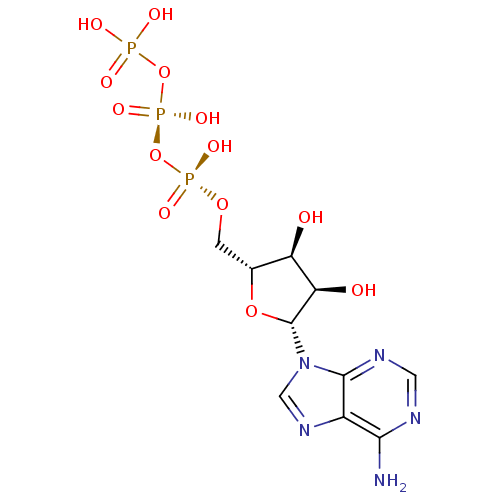 (ADENOSINE TRIPHOSPHATE | ATP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131060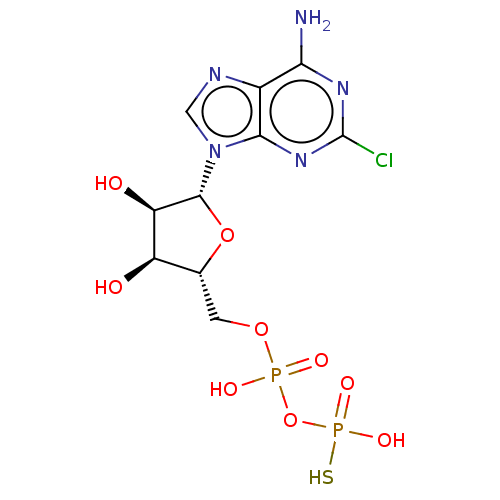 (CHEMBL3634186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131061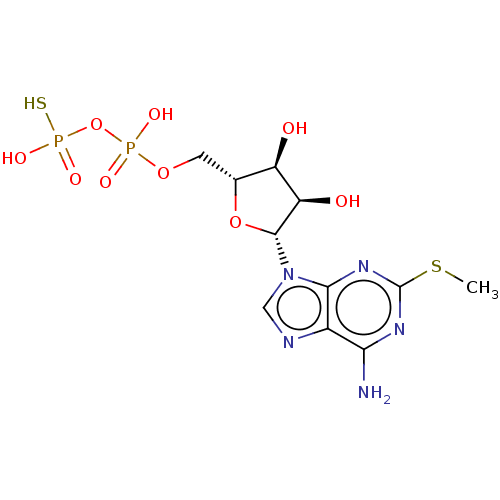 (CHEMBL3634185) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131062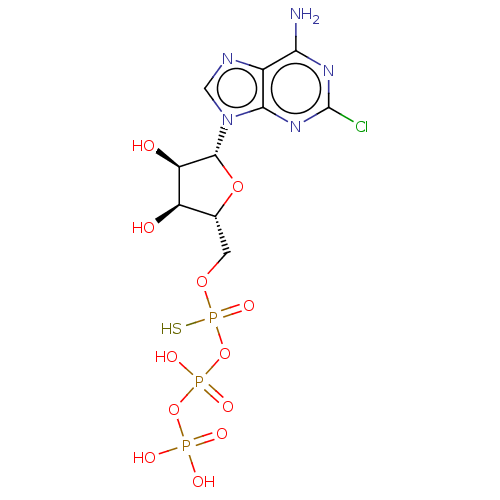 (CHEMBL3634184) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131064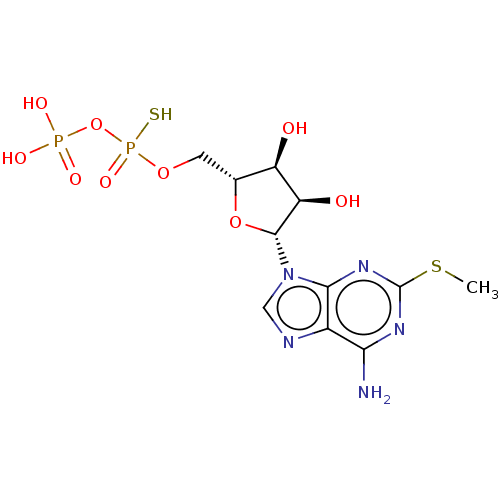 (CHEMBL3634182) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131059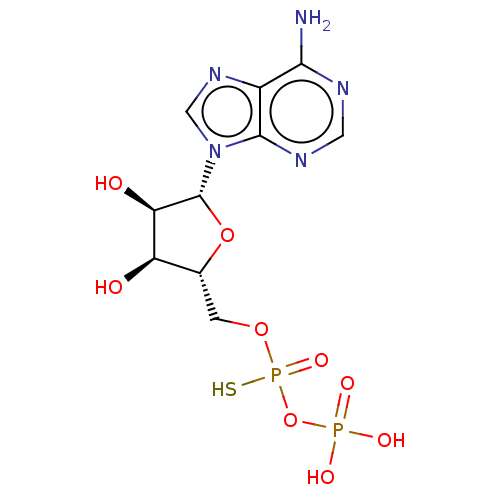 (CHEMBL575257) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118230 (5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118242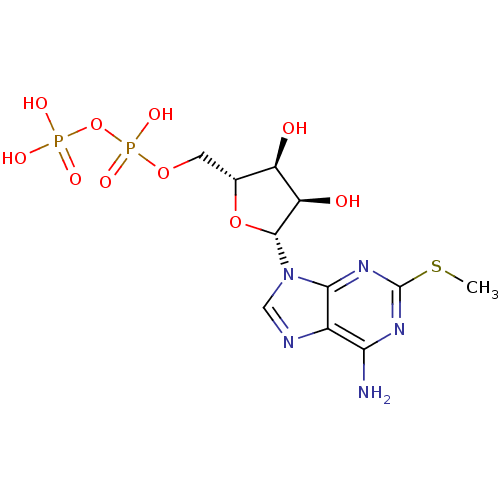 (((2R,3S,4R,5R)-5-(6-amino-2-(methylthio)-9H-purin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131060 (CHEMBL3634186) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131061 (CHEMBL3634185) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131062 (CHEMBL3634184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131064 (CHEMBL3634182) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50368125 (ADENOSINE DIPHOSPHATE | ADP) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50118230 (5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADEN...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131064 (CHEMBL3634182) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131059 (CHEMBL575257) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas... | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131062 (CHEMBL3634184) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131059 (CHEMBL575257) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131059 (CHEMBL575257) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against Cathepsin B activity | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against Cathepsin B activity | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131062 (CHEMBL3634184) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 880 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against Cathepsin B activity | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50131064 (CHEMBL3634182) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibition of [3H]-U-69,593 binding to Opioid receptor kappa 1 | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 11 (Homo sapiens (Human)) | BDBM50019292 (CHEMBL3289393) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y11 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50366480 (ADENOSINE TRIPHOSPHATE | ATP) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 850 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonist activity at human P2Y1 receptor expressed in human 1321N1 cells assessed as increase of intracellular calcium level after 30 mins using fura-... | J Med Chem 57: 4677-91 (2014) Article DOI: 10.1021/jm500196c BindingDB Entry DOI: 10.7270/Q2QN68C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50131063 (CHEMBL3634183) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-2 binding | J Med Chem 58: 8427-43 (2015) Article DOI: 10.1021/acs.jmedchem.5b00575 BindingDB Entry DOI: 10.7270/Q2736SRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||