 Found 27 hits with Last Name = 'kessel' and Initial = 'd'
Found 27 hits with Last Name = 'kessel' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sulfotransferase 1E1
(Bos taurus) | BDBM50404964
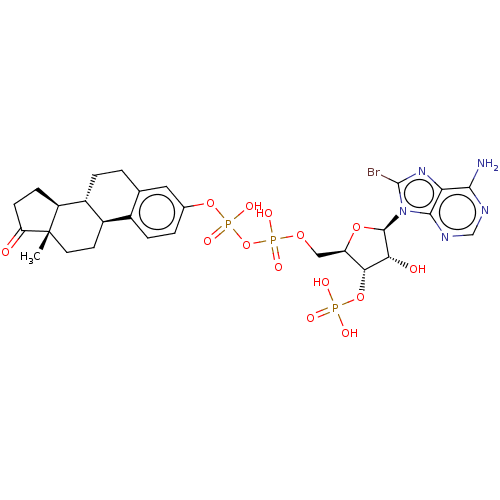
(CHEMBL2367720)Show SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(OP(O)(=O)OP(O)(=O)OC[C@H]4O[C@H]([C@H](O)[C@@H]4OP(O)(O)=O)n4c(Br)nc5c(N)ncnc45)cc3CC[C@@]21[H] Show InChI InChI=1S/C28H35BrN5O14P3/c1-28-9-8-16-15-5-3-14(10-13(15)2-4-17(16)18(28)6-7-20(28)35)46-51(42,43)48-50(40,41)44-11-19-23(47-49(37,38)39)22(36)26(45-19)34-25-21(33-27(34)29)24(30)31-12-32-25/h3,5,10,12,16-19,22-23,26,36H,2,4,6-9,11H2,1H3,(H,40,41)(H,42,43)(H2,30,31,32)(H2,37,38,39)/t16-,17-,18+,19-,22-,23-,26-,28+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase and represented as molt/4F kinase. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50028411
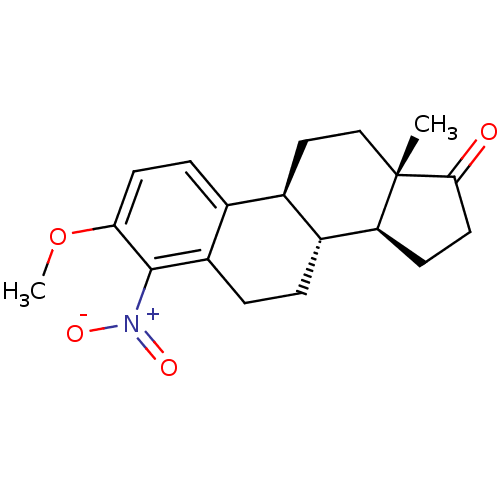
(3-Methoxy-13-methyl-4-nitro-6,7,8,9,11,12,13,14,15...)Show SMILES COc1ccc2[C@H]3CC[C@@]4(C)[C@@H](CCC4=O)[C@@H]3CCc2c1[N+]([O-])=O Show InChI InChI=1S/C19H23NO4/c1-19-10-9-12-11-5-7-16(24-2)18(20(22)23)14(11)4-3-13(12)15(19)6-8-17(19)21/h5,7,12-13,15H,3-4,6,8-10H2,1-2H3/t12-,13-,15+,19+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50367921
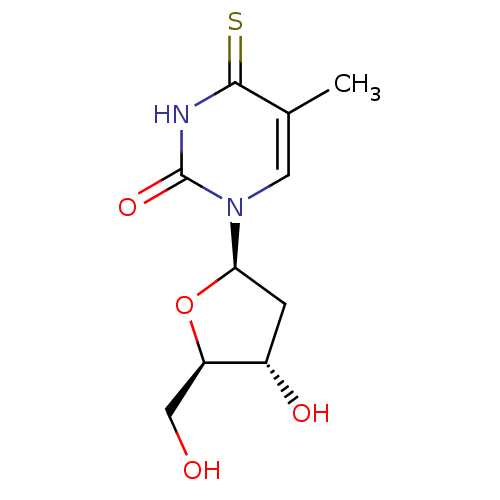
(CHEMBL1790740)Show SMILES Cc1cn([C@H]2C[C@H](O)[C@@H](CO)O2)c(=O)[nH]c1=S Show InChI InChI=1S/C10H14N2O4S/c1-5-3-12(10(15)11-9(5)17)8-2-6(14)7(4-13)16-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,17)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Iinhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50367130

(CHEMBL1628097)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OCCOC[C@H]3OC([C@H](O)[C@@H]3O)n3cnc5c(N)ncnc35)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C30H36N6O8/c1-30-9-8-16-15-4-6-20(24(36(40)41)18(15)3-2-17(16)19(30)5-7-22(30)37)43-11-10-42-12-21-25(38)26(39)29(44-21)35-14-34-23-27(31)32-13-33-28(23)35/h4,6,13-14,16-17,19,21,25-26,29,38-39H,2-3,5,7-12H2,1H3,(H2,31,32,33)/t16-,17-,19+,21-,25-,26-,29?,30+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50367920
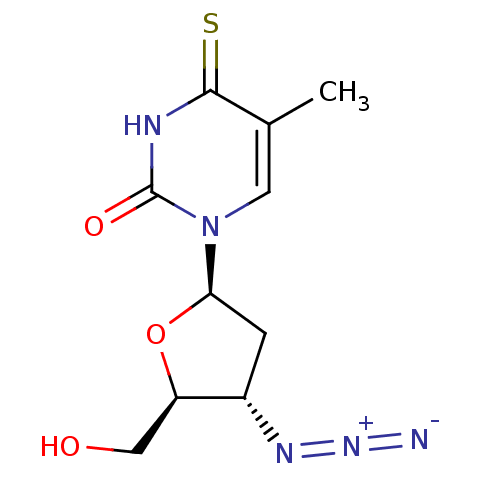
(CHEMBL1790742)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=S Show InChI InChI=1S/C10H13N5O3S/c1-5-3-15(10(17)12-9(5)19)8-2-6(13-14-11)7(4-16)18-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,19)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50367129

(CHEMBL1628095)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OCCCOC[C@H]3OC([C@H](O)[C@@H]3O)n3cnc5c(N)ncnc35)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C31H38N6O8/c1-31-10-9-17-16-5-7-21(25(37(41)42)19(16)4-3-18(17)20(31)6-8-23(31)38)44-12-2-11-43-13-22-26(39)27(40)30(45-22)36-15-35-24-28(32)33-14-34-29(24)36/h5,7,14-15,17-18,20,22,26-27,30,39-40H,2-4,6,8-13H2,1H3,(H2,32,33,34)/t17-,18-,20+,22-,26-,27-,30?,31+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50404963

(CHEMBL2067995)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OCCCCn3cnc5c(N)ncnc35)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C27H32N6O4/c1-27-11-10-17-16-6-8-21(24(33(35)36)19(16)5-4-18(17)20(27)7-9-22(27)34)37-13-3-2-12-32-15-31-23-25(28)29-14-30-26(23)32/h6,8,14-15,17-18,20H,2-5,7,9-13H2,1H3,(H2,28,29,30)/t17-,18-,20+,27+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50367131

(CHEMBL1628164)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OCCCn3cnc5c(N)ncnc35)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C26H30N6O4/c1-26-10-9-16-15-5-7-20(36-12-2-11-31-14-30-22-24(27)28-13-29-25(22)31)23(32(34)35)18(15)4-3-17(16)19(26)6-8-21(26)33/h5,7,13-14,16-17,19H,2-4,6,8-12H2,1H3,(H2,27,28,29)/t16-,17-,19+,26+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50145605

(4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...)Show InChI InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase and represented as molt/4F kinase. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013115
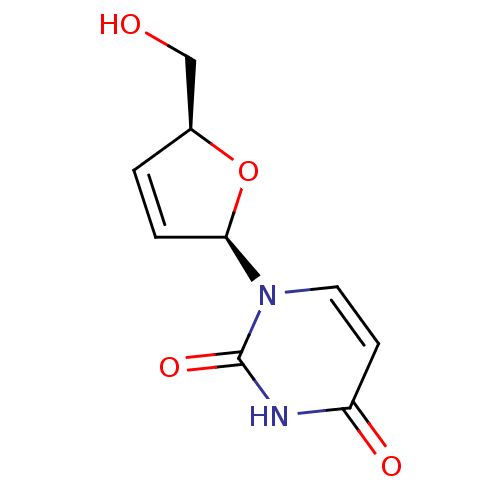
(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES OC[C@H]1O[C@H](C=C1)n1ccc(=O)[nH]c1=O |c:5| Show InChI InChI=1S/C9H10N2O4/c12-5-6-1-2-8(15-6)11-4-3-7(13)10-9(11)14/h1-4,6,8,12H,5H2,(H,10,13,14)/t6-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013114
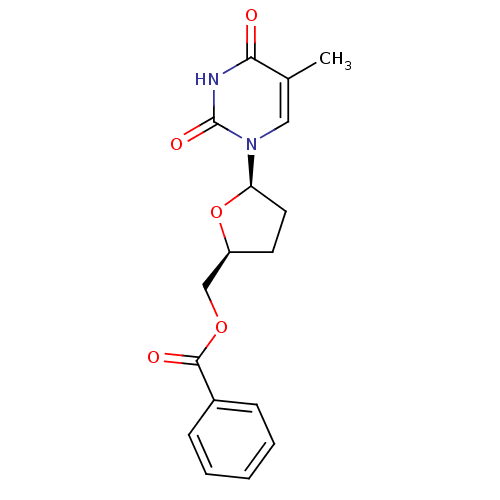
(Benzoic acid 5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-...)Show SMILES Cc1cn([C@H]2CC[C@@H](COC(=O)c3ccccc3)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C17H18N2O5/c1-11-9-19(17(22)18-15(11)20)14-8-7-13(24-14)10-23-16(21)12-5-3-2-4-6-12/h2-6,9,13-14H,7-8,10H2,1H3,(H,18,20,22)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase and represented as molt/4F kinase in two references. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013111
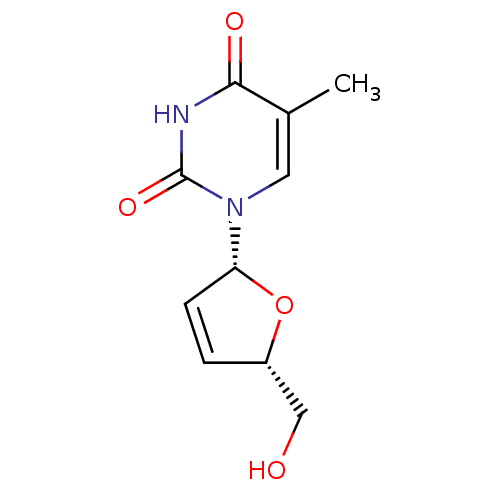
(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013114

(Benzoic acid 5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-...)Show SMILES Cc1cn([C@H]2CC[C@@H](COC(=O)c3ccccc3)O2)c(=O)[nH]c1=O Show InChI InChI=1S/C17H18N2O5/c1-11-9-19(17(22)18-15(11)20)14-8-7-13(24-14)10-23-16(21)12-5-3-2-4-6-12/h2-6,9,13-14H,7-8,10H2,1H3,(H,18,20,22)/t13-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013111

(1-((2R,5S)-5-(hydroxymethyl)-2,5-dihydrofuran-2-yl...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=O |r,c:9| Show InChI InChI=1S/C10H12N2O4/c1-6-4-12(10(15)11-9(6)14)8-3-2-7(5-13)16-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,15)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013116
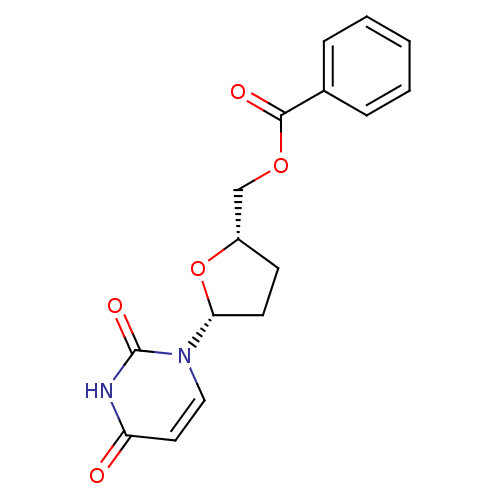
(Benzoic acid 5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin...)Show SMILES O=C(OC[C@@H]1CC[C@@H](O1)n1ccc(=O)[nH]c1=O)c1ccccc1 Show InChI InChI=1S/C16H16N2O5/c19-13-8-9-18(16(21)17-13)14-7-6-12(23-14)10-22-15(20)11-4-2-1-3-5-11/h1-5,8-9,12,14H,6-7,10H2,(H,17,19,21)/t12-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit deoxycytidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50404966
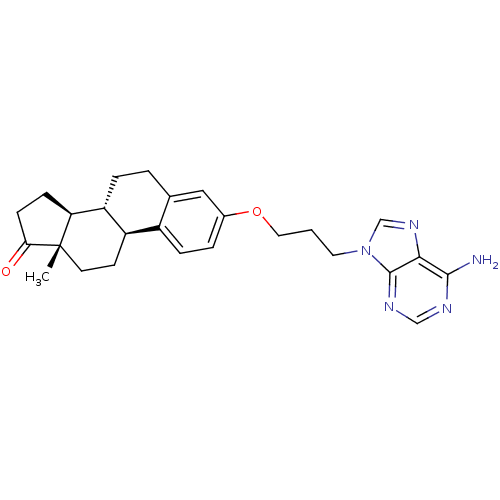
(CHEMBL2067996)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OCCCn5cnc6c(N)ncnc56)ccc34)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C26H31N5O2/c1-26-10-9-19-18-6-4-17(13-16(18)3-5-20(19)21(26)7-8-22(26)32)33-12-2-11-31-15-30-23-24(27)28-14-29-25(23)31/h4,6,13-15,19-21H,2-3,5,7-12H2,1H3,(H2,27,28,29)/t19-,20-,21+,26+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Sulfotransferase 1E1
(Bos taurus) | BDBM50404965

(CHEMBL2067994)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OCCn3cnc5c(N)ncnc35)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C25H28N6O4/c1-25-9-8-15-14-4-6-19(35-11-10-30-13-29-21-23(26)27-12-28-24(21)30)22(31(33)34)17(14)3-2-16(15)18(25)5-7-20(25)32/h4,6,12-13,15-16,18H,2-3,5,7-11H2,1H3,(H2,26,27,28)/t15-,16-,18+,25+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of bovine estrogen sulfotransferase by the compound |
J Med Chem 26: 162-6 (1983)
BindingDB Entry DOI: 10.7270/Q22F7P13 |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013116

(Benzoic acid 5-(2,4-dioxo-3,4-dihydro-2H-pyrimidin...)Show SMILES O=C(OC[C@@H]1CC[C@@H](O1)n1ccc(=O)[nH]c1=O)c1ccccc1 Show InChI InChI=1S/C16H16N2O5/c19-13-8-9-18(16(21)17-13)14-7-6-12(23-14)10-22-15(20)11-4-2-1-3-5-11/h1-5,8-9,12,14H,6-7,10H2,(H,17,19,21)/t12-,14+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase and represented as molt/4F kinase. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50145605

(4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...)Show InChI InChI=1S/C9H13N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h3-4,6,8,13H,1-2,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit deoxycytidine kinase and represented as molt/4F kinase in two references. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013119
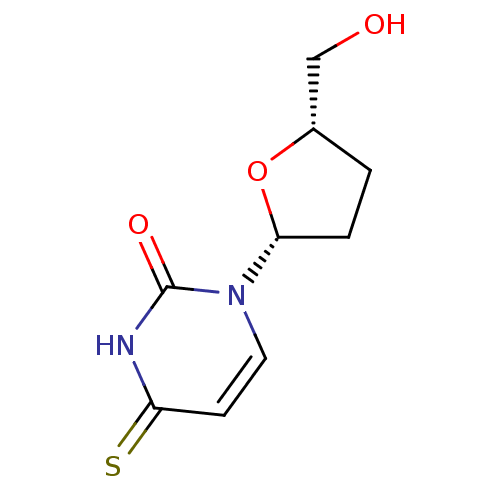
(1-(5-Hydroxymethyl-tetrahydro-furan-2-yl)-4-thioxo...)Show InChI InChI=1S/C9H12N2O3S/c12-5-6-1-2-8(14-6)11-4-3-7(15)10-9(11)13/h3-4,6,8,12H,1-2,5H2,(H,10,13,15)/t6-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.28E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013109
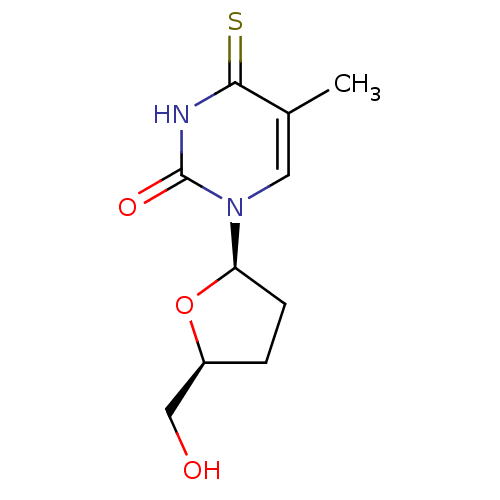
(1-(5-Hydroxymethyl-tetrahydro-furan-2-yl)-5-methyl...)Show InChI InChI=1S/C10H14N2O3S/c1-6-4-12(10(14)11-9(6)16)8-3-2-7(5-13)15-8/h4,7-8,13H,2-3,5H2,1H3,(H,11,14,16)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013118
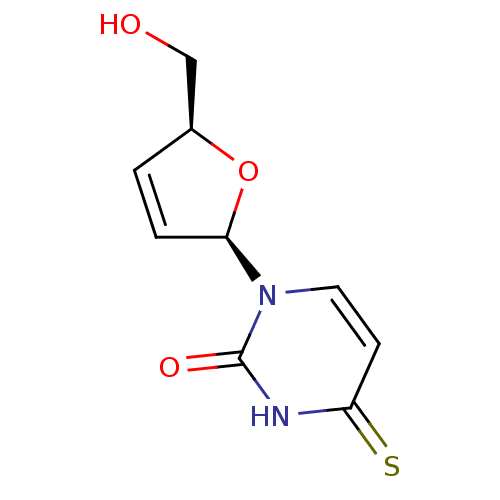
(1-(5-Hydroxymethyl-2,5-dihydro-furan-2-yl)-4-thiox...)Show SMILES OC[C@H]1O[C@H](C=C1)n1ccc(=S)[nH]c1=O |c:5| Show InChI InChI=1S/C9H10N2O3S/c12-5-6-1-2-8(14-6)11-4-3-7(15)10-9(11)13/h1-4,6,8,12H,5H2,(H,10,13,15)/t6-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.69E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Thymidine kinase, cytosolic
(Homo sapiens (Human)) | BDBM50013110
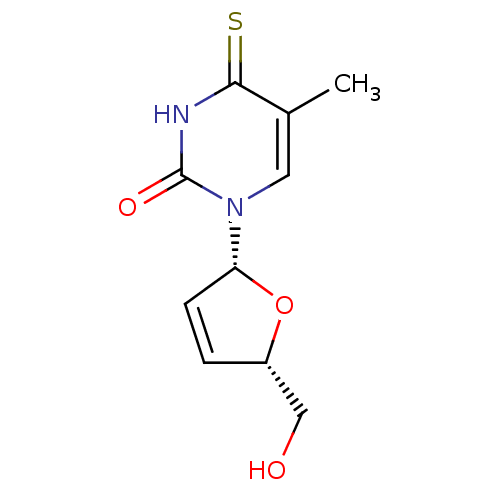
(1-(5-Hydroxymethyl-2,5-dihydro-furan-2-yl)-5-methy...)Show SMILES Cc1cn([C@@H]2O[C@H](CO)C=C2)c(=O)[nH]c1=S |c:9| Show InChI InChI=1S/C10H12N2O3S/c1-6-4-12(10(14)11-9(6)16)8-3-2-7(5-13)15-8/h2-4,7-8,13H,5H2,1H3,(H,11,14,16)/t7-,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.69E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit thymus thymidine kinase |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50421873
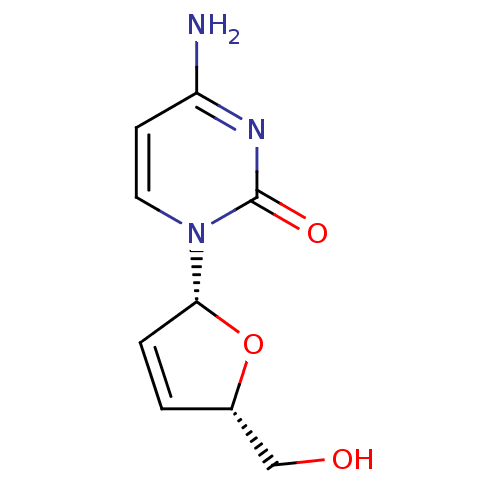
(CHEMBL2115278)Show SMILES Nc1ccn([C@@H]2O[C@H](CO)C=C2)c(=O)n1 |r,c:10| Show InChI InChI=1S/C9H11N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h1-4,6,8,13H,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit deoxycytidine kinase and represented as molt/4F kinase. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |
Deoxycytidine kinase
(Homo sapiens (Human)) | BDBM50421873

(CHEMBL2115278)Show SMILES Nc1ccn([C@@H]2O[C@H](CO)C=C2)c(=O)n1 |r,c:10| Show InChI InChI=1S/C9H11N3O3/c10-7-3-4-12(9(14)11-7)8-2-1-6(5-13)15-8/h1-4,6,8,13H,5H2,(H2,10,11,14)/t6-,8+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Michigan Cancer Foundation
Curated by ChEMBL
| Assay Description
Inhibitory affect against rabbit deoxycytidine kinase and represented as molt/4F kinase. |
J Med Chem 33: 258-63 (1990)
BindingDB Entry DOI: 10.7270/Q2P26ZQS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data