 Found 2074 hits with Last Name = 'kie?-kononowicz' and Initial = 'k'
Found 2074 hits with Last Name = 'kie?-kononowicz' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50494549
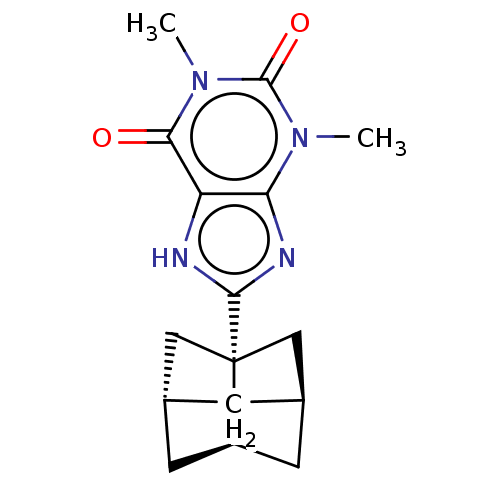
(CHEMBL3093327)Show SMILES [H][C@@]12C[C@@]3([H])C[C@]([H])(C[C@]3(C1)c1nc3n(C)c(=O)n(C)c(=O)c3[nH]1)C2 |r,wD:9.12,6.6,3.3,1.0,TLB:5:6:10:2.3,THB:5:3:10:24.6.8,(14.09,-14.42,;12.36,-15.15,;12.34,-13.62,;10.87,-13.16,;12.72,-13.48,;13.43,-13.25,;13.03,-14.75,;14.92,-14.74,;11.3,-15.18,;9.98,-14.41,;10.9,-15.65,;8.44,-14.44,;7.53,-15.7,;6.07,-15.24,;4.75,-16.03,;4.75,-17.57,;3.4,-15.27,;2.07,-16.06,;3.39,-13.73,;2.04,-12.98,;4.71,-12.95,;4.68,-11.41,;6.05,-13.7,;7.5,-13.21,;13.44,-16.24,)| Show InChI InChI=1S/C16H20N4O2/c1-19-12-11(13(21)20(2)15(19)22)17-14(18-12)16-6-8-3-9(7-16)5-10(16)4-8/h8-10H,3-7H2,1-2H3,(H,17,18)/t8-,9-,10-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Binding affinity to rat adenosine A1 receptor |
Bioorg Med Chem 21: 7435-52 (2013)
Article DOI: 10.1016/j.bmc.2013.09.044
BindingDB Entry DOI: 10.7270/Q2TT4TWF |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50535225
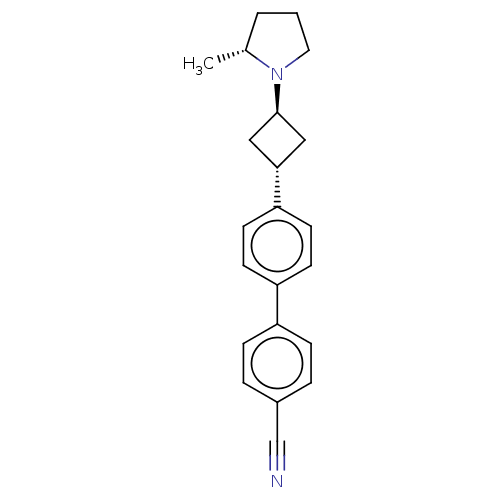
(CHEMBL4516622)Show SMILES C[C@@H]1CCCN1[C@H]1C[C@@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r,wU:8.11,1.0,wD:6.6,(34.83,-7.94,;35.15,-9.44,;34.11,-10.59,;34.88,-11.92,;36.39,-11.6,;36.55,-10.07,;37.88,-9.3,;39.37,-9.7,;39.77,-8.21,;38.28,-7.81,;41.1,-7.45,;41.1,-5.9,;42.43,-5.13,;43.77,-5.9,;43.77,-7.45,;42.43,-8.22,;45.09,-5.12,;46.43,-5.89,;47.76,-5.12,;47.75,-3.58,;46.41,-2.81,;45.08,-3.59,;49.08,-2.8,;50.41,-2.03,)| Show InChI InChI=1S/C22H24N2/c1-16-3-2-12-24(16)22-13-21(14-22)20-10-8-19(9-11-20)18-6-4-17(15-23)5-7-18/h4-11,16,21-22H,2-3,12-14H2,1H3/t16-,21-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Rattus norvegicus) | BDBM50455533
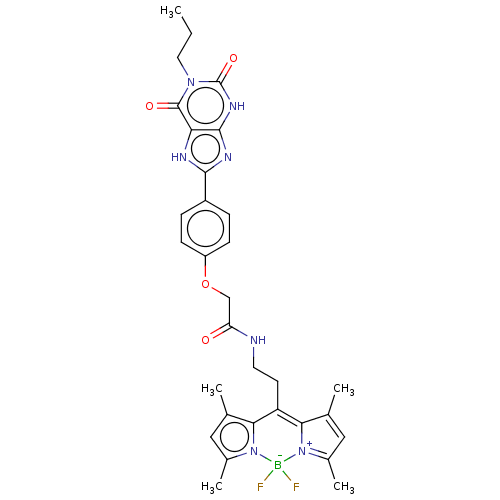
(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant rat adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation countin... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-prazosin from alpha1-adrenergic receptor in rat cerebral cortex membranes after 30 mins by scintillation counting |
Bioorg Med Chem 19: 1349-60 (2011)
Article DOI: 10.1016/j.bmc.2010.11.051
BindingDB Entry DOI: 10.7270/Q2TT4TS3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110

(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H3 receptor |
Bioorg Med Chem 25: 2701-2712 (2017)
Article DOI: 10.1016/j.bmc.2017.03.031
BindingDB Entry DOI: 10.7270/Q2833VPK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human H3 receptor |
Eur J Med Chem 152: 223-234 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.043
BindingDB Entry DOI: 10.7270/Q2697632 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158595
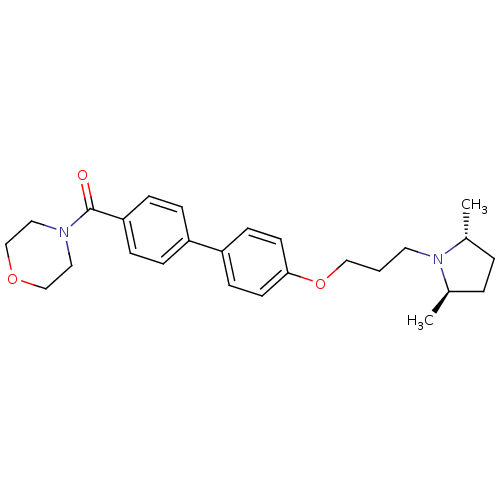
((4'-(3-((2R,5R)-2,5-dimethylpyrrolidin-1-yl)propox...)Show SMILES C[C@@H]1CC[C@@H](C)N1CCCOc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCOCC1 |r| Show InChI InChI=1S/C26H34N2O3/c1-20-4-5-21(2)28(20)14-3-17-31-25-12-10-23(11-13-25)22-6-8-24(9-7-22)26(29)27-15-18-30-19-16-27/h6-13,20-21H,3-5,14-19H2,1-2H3/t20-,21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Binding affinity to human H3R expressed in rat C6 cells |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM28583
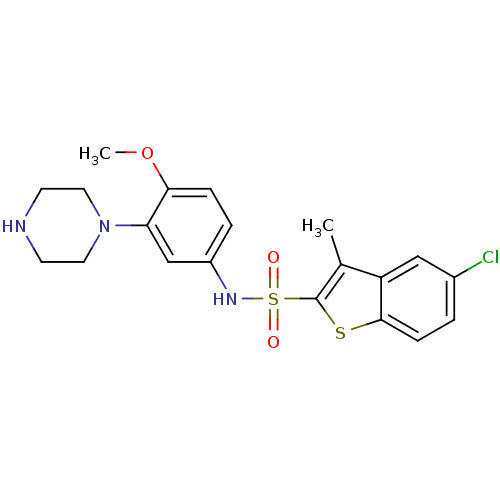
(5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...)Show SMILES COc1ccc(NS(=O)(=O)c2sc3ccc(Cl)cc3c2C)cc1N1CCNCC1 Show InChI InChI=1S/C20H22ClN3O3S2/c1-13-16-11-14(21)3-6-19(16)28-20(13)29(25,26)23-15-4-5-18(27-2)17(12-15)24-9-7-22-8-10-24/h3-6,11-12,22-23H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells after 1 hr by liquid scintillation counting method |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50494549

(CHEMBL3093327)Show SMILES [H][C@@]12C[C@@]3([H])C[C@]([H])(C[C@]3(C1)c1nc3n(C)c(=O)n(C)c(=O)c3[nH]1)C2 |r,wD:9.12,6.6,3.3,1.0,TLB:5:6:10:2.3,THB:5:3:10:24.6.8,(14.09,-14.42,;12.36,-15.15,;12.34,-13.62,;10.87,-13.16,;12.72,-13.48,;13.43,-13.25,;13.03,-14.75,;14.92,-14.74,;11.3,-15.18,;9.98,-14.41,;10.9,-15.65,;8.44,-14.44,;7.53,-15.7,;6.07,-15.24,;4.75,-16.03,;4.75,-17.57,;3.4,-15.27,;2.07,-16.06,;3.39,-13.73,;2.04,-12.98,;4.71,-12.95,;4.68,-11.41,;6.05,-13.7,;7.5,-13.21,;13.44,-16.24,)| Show InChI InChI=1S/C16H20N4O2/c1-19-12-11(13(21)20(2)15(19)22)17-14(18-12)16-6-8-3-9(7-16)5-10(16)4-8/h8-10H,3-7H2,1-2H3,(H,17,18)/t8-,9-,10-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A1 receptor |
Bioorg Med Chem 21: 7435-52 (2013)
Article DOI: 10.1016/j.bmc.2013.09.044
BindingDB Entry DOI: 10.7270/Q2TT4TWF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50419052

(SB-399885)Show SMILES COc1ccc(cc1N1CCNCC1)S(=O)(=O)Nc1cc(Cl)cc(Cl)c1OC Show InChI InChI=1S/C18H21Cl2N3O4S/c1-26-17-4-3-13(11-16(17)23-7-5-21-6-8-23)28(24,25)22-15-10-12(19)9-14(20)18(15)27-2/h3-4,9-11,21-22H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.724 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Technology and Biotechnology of Drugs, Jagiellonian University, Medical College, Medyczna 9, PL 30-688 Kraków, Poland. Electronic address: dlazewska@cm-uj.krakow.pl.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HeLa cells |
Eur J Med Chem 135: 117-124 (2017)
Article DOI: 10.1016/j.ejmech.2017.04.033
BindingDB Entry DOI: 10.7270/Q2QN696C |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] raclopride from human recombinant D2L receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Mus musculus) | BDBM50514105
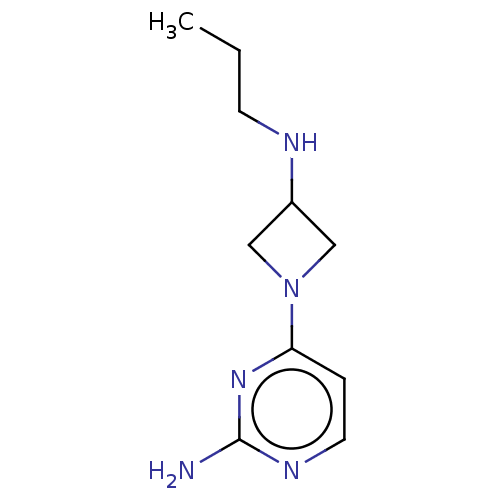
(CHEMBL4441731)Show SMILES O.OC(=O)\C=C\C(O)=O.CCCNC1CN(C1)c1ccnc(N)n1 Show InChI InChI=1S/C10H17N5/c1-2-4-12-8-6-15(7-8)9-3-5-13-10(11)14-9/h3,5,8,12H,2,4,6-7H2,1H3,(H2,11,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis |
J Med Chem 62: 10848-10866 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01462
BindingDB Entry DOI: 10.7270/Q2M61PKJ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50146835
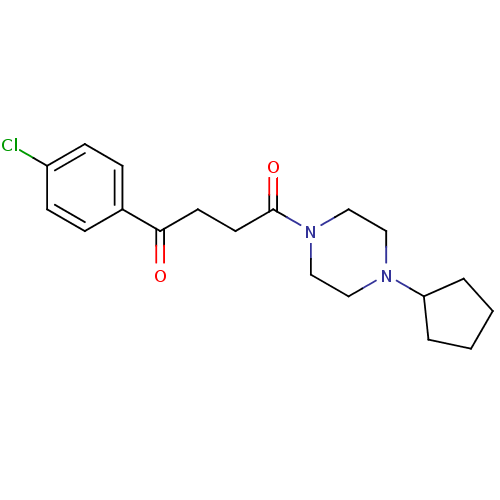
(1-(4-Chloro-phenyl)-4-(4-cyclopentyl-piperazin-1-y...)Show InChI InChI=1S/C19H25ClN2O2/c20-16-7-5-15(6-8-16)18(23)9-10-19(24)22-13-11-21(12-14-22)17-3-1-2-4-17/h5-8,17H,1-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Mus musculus) | BDBM50455533

(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant mouse adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM30712
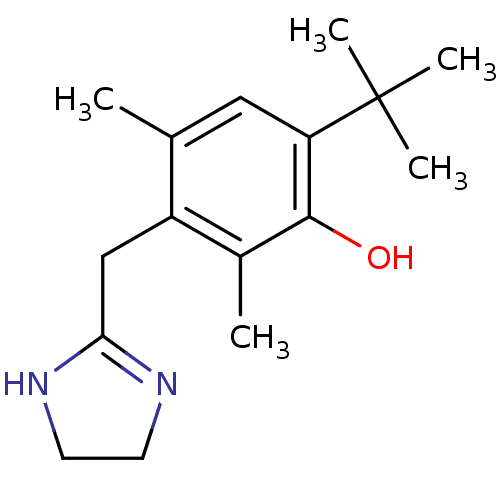
(6-tert-butyl-3-(2-imidazolin-2-ylmethyl)-2,4-dimet...)Show InChI InChI=1S/C16H24N2O/c1-10-8-13(16(3,4)5)15(19)11(2)12(10)9-14-17-6-7-18-14/h8,19H,6-7,9H2,1-5H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-clonidine from alpha2-adrenergic receptor in rat brain cortex after 30 mins by Microbeta scintillation counting method |
Eur J Med Chem 147: 102-114 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.093
BindingDB Entry DOI: 10.7270/Q29P346S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455533

(CHEMBL4205635)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:27,30,33| Show InChI InChI=1S/C31H34BF2N7O4/c1-6-13-39-30(43)25-29(38-31(39)44)37-28(36-25)21-7-9-22(10-8-21)45-16-24(42)35-12-11-23-26-17(2)14-19(4)40(26)32(33,34)41-20(5)15-18(3)27(23)41/h7-10,14-15H,6,11-13,16H2,1-5H3,(H,35,42)(H,36,37)(H,38,44) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50533199
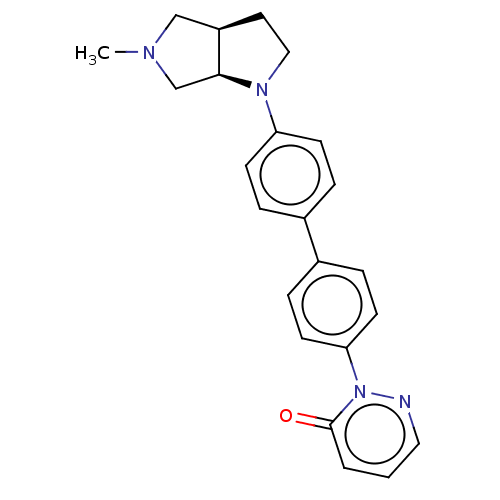
(CHEMBL4527011)Show SMILES [H][C@]12CCN(c3ccc(cc3)-c3ccc(cc3)-n3ncccc3=O)[C@@]1([H])CN(C)C2 |r| Show InChI InChI=1S/C23H24N4O/c1-25-15-19-12-14-26(22(19)16-25)20-8-4-17(5-9-20)18-6-10-21(11-7-18)27-23(28)3-2-13-24-27/h2-11,13,19,22H,12,14-16H2,1H3/t19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054
BindingDB Entry DOI: 10.7270/Q2XD155T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50533199

(CHEMBL4527011)Show SMILES [H][C@]12CCN(c3ccc(cc3)-c3ccc(cc3)-n3ncccc3=O)[C@@]1([H])CN(C)C2 |r| Show InChI InChI=1S/C23H24N4O/c1-25-15-19-12-14-26(22(19)16-25)20-8-4-17(5-9-20)18-6-10-21(11-7-18)27-23(28)3-2-13-24-27/h2-11,13,19,22H,12,14-16H2,1H3/t19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Antagonist activity at human H3R expressed in rat C6 cells incubated for 20 mins by [35S]GTPgammaS binding assay |
Bioorg Med Chem 25: 5341-5354 (2017)
Article DOI: 10.1016/j.bmc.2017.07.058
BindingDB Entry DOI: 10.7270/Q29W0K0D |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50533199

(CHEMBL4527011)Show SMILES [H][C@]12CCN(c3ccc(cc3)-c3ccc(cc3)-n3ncccc3=O)[C@@]1([H])CN(C)C2 |r| Show InChI InChI=1S/C23H24N4O/c1-25-15-19-12-14-26(22(19)16-25)20-8-4-17(5-9-20)18-6-10-21(11-7-18)27-23(28)3-2-13-24-27/h2-11,13,19,22H,12,14-16H2,1H3/t19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]Nalpha-methylhistamine from full-length human histamine H3 receptor expressed in HEK cells after 30 mins by liquid scintillation ... |
Bioorg Med Chem Lett 26: 4140-5 (2016)
Article DOI: 10.1016/j.bmcl.2016.04.054
BindingDB Entry DOI: 10.7270/Q2XD155T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50268815
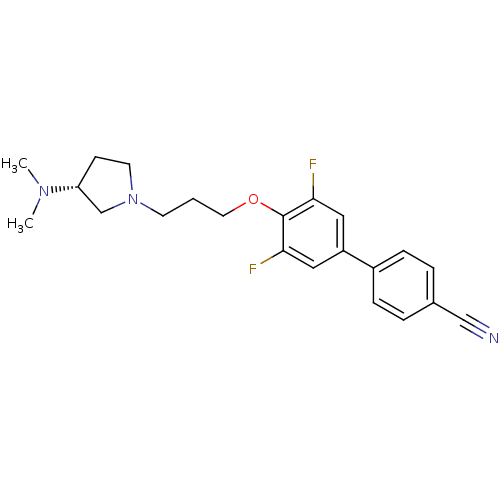
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2c(F)cc(cc2F)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H25F2N3O/c1-26(2)19-8-10-27(15-19)9-3-11-28-22-20(23)12-18(13-21(22)24)17-6-4-16(14-25)5-7-17/h4-7,12-13,19H,3,8-11,15H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709
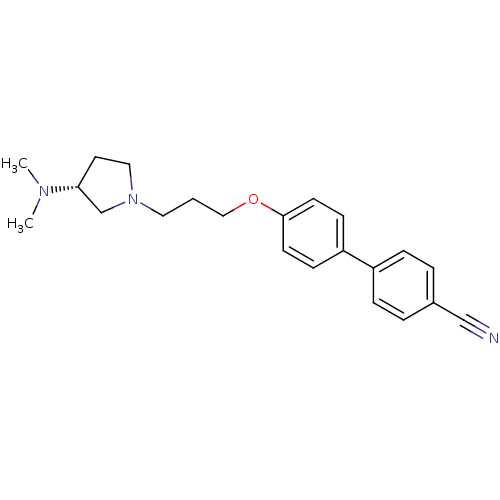
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(R)alpha-MeHA from rat brain H3 receptor |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50247053

(1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...)Show InChI InChI=1S/C17H26ClNO/c18-17-9-7-16(8-10-17)6-4-14-20-15-5-13-19-11-2-1-3-12-19/h7-10H,1-6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-iodoproxyfan from human striatal full length H3 receptor after 60 mins by gamma counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50274767
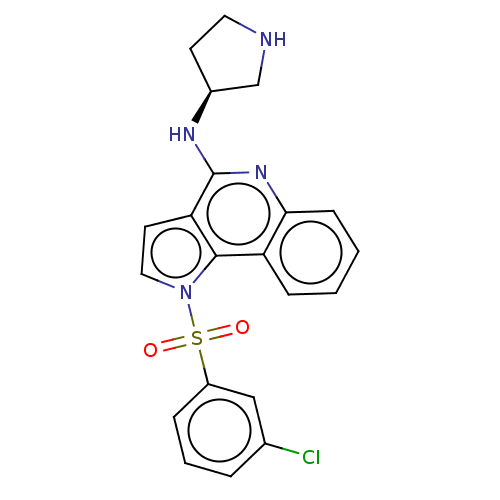
(CHEMBL4125735)Show SMILES Clc1cccc(c1)S(=O)(=O)n1ccc2c(N[C@H]3CCNC3)nc3ccccc3c12 |r| Show InChI InChI=1S/C21H19ClN4O2S/c22-14-4-3-5-16(12-14)29(27,28)26-11-9-18-20(26)17-6-1-2-7-19(17)25-21(18)24-15-8-10-23-13-15/h1-7,9,11-12,15,23H,8,10,13H2,(H,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from human 5-HT6R expressed in human HEK293 cells incubated for 1 hr by radioligand binding assay |
Eur J Med Chem 178: 740-751 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.022
BindingDB Entry DOI: 10.7270/Q22F7RSS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562553
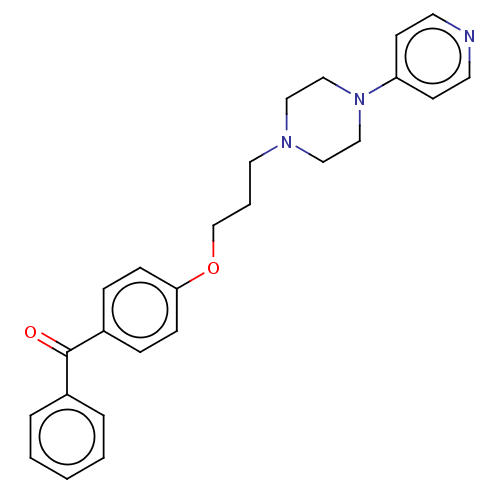
(CHEMBL4759150)Show SMILES O=C(c1ccccc1)c1ccc(OCCCN2CCN(CC2)c2ccncc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562541

(CHEMBL4783744) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50514105

(CHEMBL4441731)Show SMILES O.OC(=O)\C=C\C(O)=O.CCCNC1CN(C1)c1ccnc(N)n1 Show InChI InChI=1S/C10H17N5/c1-2-4-12-8-6-15(7-8)9-3-5-13-10(11)14-9/h3,5,8,12H,2,4,6-7H2,1H3,(H2,11,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis |
J Med Chem 62: 10848-10866 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01462
BindingDB Entry DOI: 10.7270/Q2M61PKJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455527
(CHEMBL4218460)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCCCc2ccccc2)cc1 Show InChI InChI=1S/C26H29N5O4/c1-2-16-31-25(33)22-24(30-26(31)34)29-23(28-22)19-11-13-20(14-12-19)35-17-21(32)27-15-7-6-10-18-8-4-3-5-9-18/h3-5,8-9,11-14H,2,6-7,10,15-17H2,1H3,(H,27,32)(H,28,29)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562552
(CHEMBL4796748)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2F)-c2ccc(cc2)C#N)C1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human H3R |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524111

(CHEMBL4452569)Show InChI InChI=1S/C13H11FIN3/c1-2-18-7-16-6-11(18)8-5-17-10-4-3-9(15)13(14)12(8)10/h3-7,17H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50438370
(CHEMBL2413101)Show SMILES OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(Nc3ccc(CC(=O)Nc4ccc(CC(=O)NCCNc5ccc([N+]([O-])=O)c6nonc56)cc4)cc3)ncnc12 |r| Show InChI InChI=1S/C34H33N11O9/c46-15-24-30(49)31(50)34(53-24)44-17-39-29-32(37-16-38-33(29)44)41-21-7-3-19(4-8-21)14-26(48)40-20-5-1-18(2-6-20)13-25(47)36-12-11-35-22-9-10-23(45(51)52)28-27(22)42-54-43-28/h1-10,16-17,24,30-31,34-35,46,49-50H,11-15H2,(H,36,47)(H,40,48)(H,37,38,41)/t24-,30-,31-,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]-ADAC from adenosine A1 receptor in rat cerebral cortex membranes after 120 mins by filter binding method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50176050
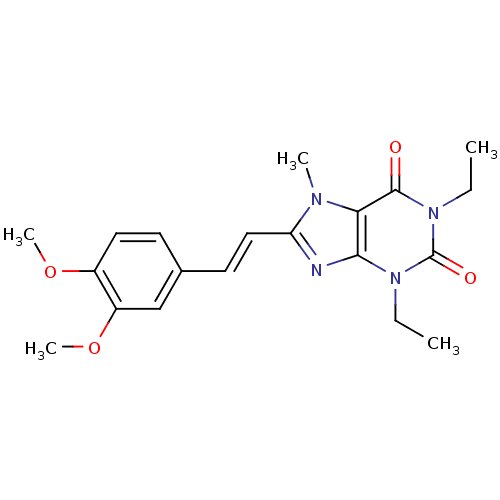
(8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...)Show SMILES CCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]CCPA from rat adenosine A2a receptor |
Bioorg Med Chem 27: 1195-1210 (2019)
Article DOI: 10.1016/j.bmc.2019.02.004
BindingDB Entry DOI: 10.7270/Q2ZS312P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50176050

(8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-1H-pu...)Show SMILES CCn1c2nc(\C=C\c3ccc(OC)c(OC)c3)n(C)c2c(=O)n(CC)c1=O Show InChI InChI=1S/C20H24N4O4/c1-6-23-18-17(19(25)24(7-2)20(23)26)22(3)16(21-18)11-9-13-8-10-14(27-4)15(12-13)28-5/h8-12H,6-7H2,1-5H3/b11-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGS21680 from adenosine A2A receptor in rat brain after 60 mins by scintillation counting analysis |
Bioorg Med Chem 21: 7435-52 (2013)
Article DOI: 10.1016/j.bmc.2013.09.044
BindingDB Entry DOI: 10.7270/Q2TT4TWF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50004518
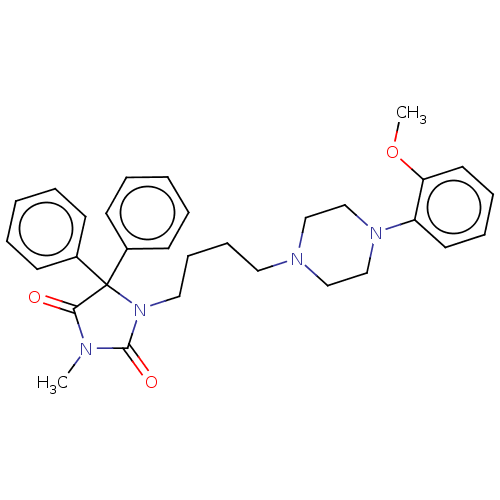
(CHEMBL2079256)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-32-29(36)31(25-13-5-3-6-14-25,26-15-7-4-8-16-26)35(30(32)37)20-12-11-19-33-21-23-34(24-22-33)27-17-9-10-18-28(27)38-2/h3-10,13-18H,11-12,19-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by microbeta scintillation counting |
Bioorg Med Chem 20: 2290-303 (2012)
Article DOI: 10.1016/j.bmc.2012.02.009
BindingDB Entry DOI: 10.7270/Q2XW4NP9 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50004518

(CHEMBL2079256)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)N(C)C(=O)C2(c2ccccc2)c2ccccc2)CC1 Show InChI InChI=1S/C31H36N4O3/c1-32-29(36)31(25-13-5-3-6-14-25,26-15-7-4-8-16-26)35(30(32)37)20-12-11-19-33-21-23-34(24-22-33)27-17-9-10-18-28(27)38-2/h3-10,13-18H,11-12,19-24H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from alpha1 adrenoceptor in rat cerebral cortex after 30 mins by microbeta scintillation counting |
Bioorg Med Chem 20: 2290-303 (2012)
Article DOI: 10.1016/j.bmc.2012.02.009
BindingDB Entry DOI: 10.7270/Q2XW4NP9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50524116
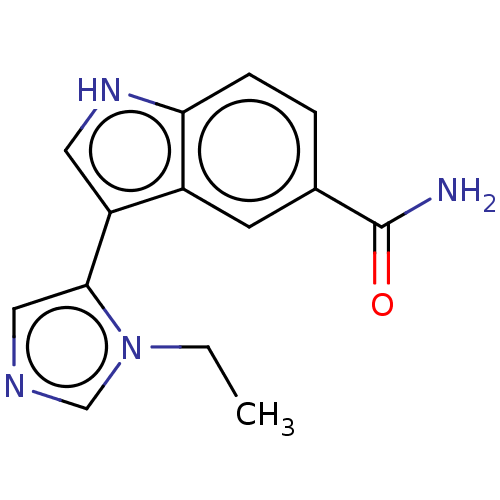
(CHEMBL4469847)Show InChI InChI=1S/C14H14N4O/c1-2-18-8-16-7-13(18)11-6-17-12-4-3-9(14(15)19)5-10(11)12/h3-8,17H,2H2,1H3,(H2,15,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50520939
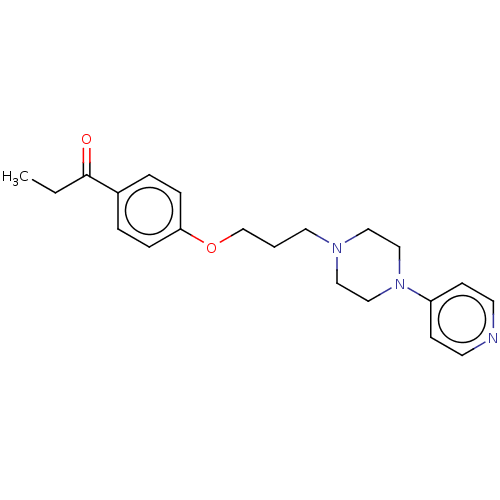
(CHEMBL4449790)Show InChI InChI=1S/C21H27N3O2/c1-2-21(25)18-4-6-20(7-5-18)26-17-3-12-23-13-15-24(16-14-23)19-8-10-22-11-9-19/h4-11H,2-3,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha-methylhistamine from full length recombinant human H3R expressed in HEK293 cell membranes after 90 mins by beta scintilla... |
Bioorg Med Chem 26: 6056-6066 (2018)
Article DOI: 10.1016/j.bmc.2018.11.010
BindingDB Entry DOI: 10.7270/Q2NV9NNX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50272040
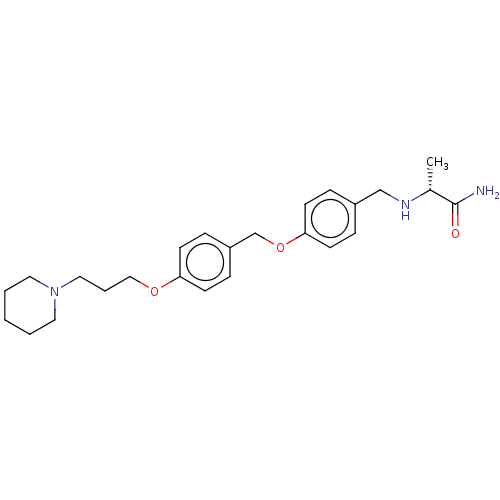
(CHEMBL4129950)Show SMILES C[C@@H](NCc1ccc(OCc2ccc(OCCCN3CCCCC3)cc2)cc1)C(N)=O |r| Show InChI InChI=1S/C25H35N3O3/c1-20(25(26)29)27-18-21-6-10-24(11-7-21)31-19-22-8-12-23(13-9-22)30-17-5-16-28-14-3-2-4-15-28/h6-13,20,27H,2-5,14-19H2,1H3,(H2,26,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Nalpha-MeHA from human H3 receptor expressed in HEK293 cell membranes by scintillation counting method |
Bioorg Med Chem 26: 2573-2585 (2018)
Article DOI: 10.1016/j.bmc.2018.04.023
BindingDB Entry DOI: 10.7270/Q2TQ641P |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50084860
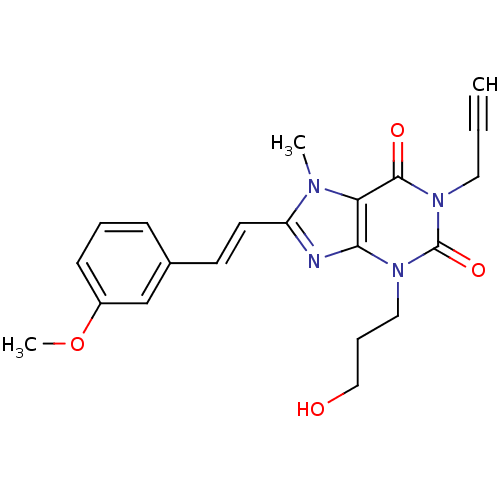
(3-(3-Hydroxy-propyl)-8-[2-(3-methoxy-phenyl)-vinyl...)Show SMILES COc1cccc(\C=C\c2nc3n(CCCO)c(=O)n(CC#C)c(=O)c3n2C)c1 Show InChI InChI=1S/C21H22N4O4/c1-4-11-25-20(27)18-19(24(21(25)28)12-6-13-26)22-17(23(18)2)10-9-15-7-5-8-16(14-15)29-3/h1,5,7-10,14,26H,6,11-13H2,2-3H3/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem 21: 7435-52 (2013)
Article DOI: 10.1016/j.bmc.2013.09.044
BindingDB Entry DOI: 10.7270/Q2TT4TWF |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50084860

(3-(3-Hydroxy-propyl)-8-[2-(3-methoxy-phenyl)-vinyl...)Show SMILES COc1cccc(\C=C\c2nc3n(CCCO)c(=O)n(CC#C)c(=O)c3n2C)c1 Show InChI InChI=1S/C21H22N4O4/c1-4-11-25-20(27)18-19(24(21(25)28)12-6-13-26)22-17(23(18)2)10-9-15-7-5-8-16(14-15)29-3/h1,5,7-10,14,26H,6,11-13H2,2-3H3/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]MSX-2 from human adenosine A2A receptor expressed in CHO cells |
Bioorg Med Chem 21: 7435-52 (2013)
Article DOI: 10.1016/j.bmc.2013.09.044
BindingDB Entry DOI: 10.7270/Q2TT4TWF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H] ketanserin from human recombinant 5-HT2A receptor expressed in CHOK1 cells after 1 hr by microbeta scintillation counting method |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50562543
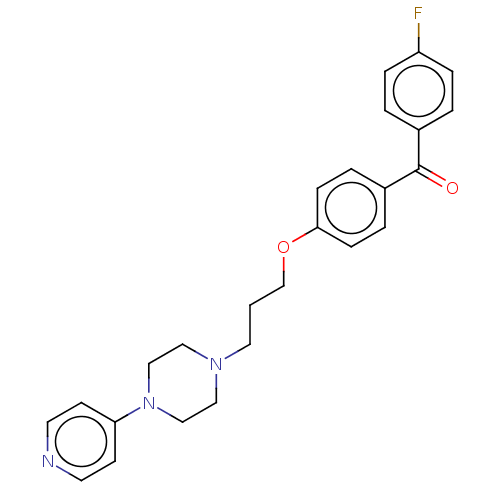
(CHEMBL4741145)Show SMILES Fc1ccc(cc1)C(=O)c1ccc(OCCCN2CCN(CC2)c2ccncc2)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]UR-PI294 from human SP-FLAG-tagged H3R expressed in HEK293T cells measured after 60 to 120 mins by liquid scintillation counting ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113041
BindingDB Entry DOI: 10.7270/Q2MP570W |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2b
(Homo sapiens (Human)) | BDBM50455526

(CHEMBL4218673)Show SMILES CCCn1c(=O)[nH]c2nc([nH]c2c1=O)-c1ccc(OCC(=O)NCCCCCC2=C3C(C)=CC(C)=[N+]3[B-](F)(F)n3c(C)cc(C)c23)cc1 |c:30,33,36| Show InChI InChI=1S/C34H40BF2N7O4/c1-6-16-42-33(46)28-32(41-34(42)47)40-31(39-28)24-11-13-25(14-12-24)48-19-27(45)38-15-9-7-8-10-26-29-20(2)17-22(4)43(29)35(36,37)44-23(5)18-21(3)30(26)44/h11-14,17-18H,6-10,15-16,19H2,1-5H3,(H,38,45)(H,39,40)(H,41,47) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [3H]PSB-603 from recombinant human adenosine A2B receptor expressed in CHO cell membranes after 75 mins by liquid scintillation count... |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50458530
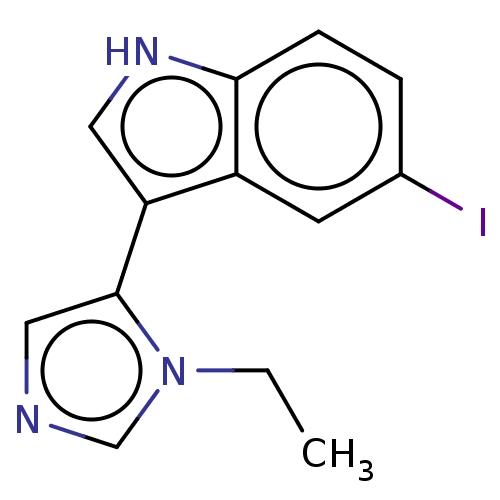
(CHEMBL4216263)Show InChI InChI=1S/C13H12IN3/c1-2-17-8-15-7-13(17)11-6-16-12-4-3-9(14)5-10(11)12/h3-8,16H,2H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human recombinant 5-HT7B receptor expressed in HEK293 cells measured after 1 hr by microbeta scintillation counting me... |
Eur J Med Chem 170: 261-275 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.017
BindingDB Entry DOI: 10.7270/Q2222Z6Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50304358
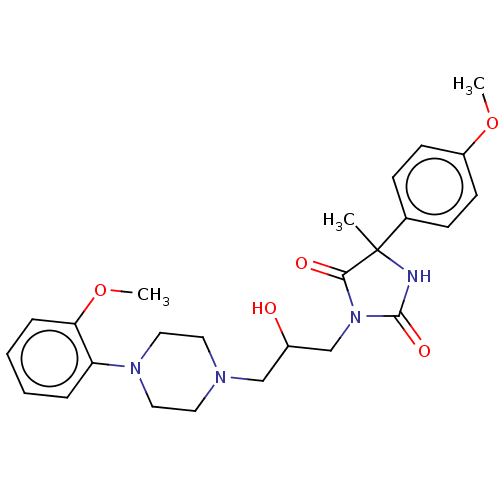
(CHEMBL4163778)Show SMILES Cl.COc1ccc(cc1)C1(C)NC(=O)N(CC(O)CN2CCN(CC2)c2ccccc2OC)C1=O Show InChI InChI=1S/C25H32N4O5.ClH/c1-25(18-8-10-20(33-2)11-9-18)23(31)29(24(32)26-25)17-19(30)16-27-12-14-28(15-13-27)21-6-4-5-7-22(21)34-3;/h4-11,19,30H,12-17H2,1-3H3,(H,26,32);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5HT7BR expressed in HEK293 cell membranes after 1 hr by Microbeta scintillation counting method |
Eur J Med Chem 147: 102-114 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.093
BindingDB Entry DOI: 10.7270/Q29P346S |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50305485
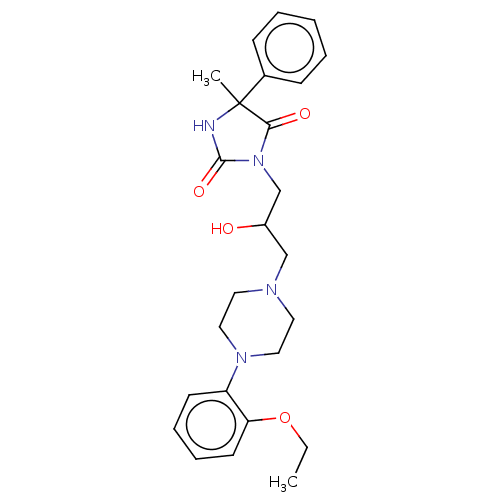
(CHEMBL4176950)Show SMILES Cl.CCOc1ccccc1N1CCN(CC(O)CN2C(=O)NC(C)(C2=O)c2ccccc2)CC1 Show InChI InChI=1S/C25H32N4O4.ClH/c1-3-33-22-12-8-7-11-21(22)28-15-13-27(14-16-28)17-20(30)18-29-23(31)25(2,26-24(29)32)19-9-5-4-6-10-19;/h4-12,20,30H,3,13-18H2,1-2H3,(H,26,32);1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College
Curated by ChEMBL
| Assay Description
Displacement of [3H]-5-CT from human 5HT7BR expressed in HEK293 cell membranes after 1 hr by Microbeta scintillation counting method |
Eur J Med Chem 147: 102-114 (2018)
Article DOI: 10.1016/j.ejmech.2018.01.093
BindingDB Entry DOI: 10.7270/Q29P346S |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50514097

(CHEMBL4466700)Show InChI InChI=1S/C8H13N5/c1-10-6-4-13(5-6)7-2-3-11-8(9)12-7/h2-3,6,10H,4-5H2,1H3,(H2,9,11,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [3H]NAMH from human H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis |
J Med Chem 62: 10848-10866 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01462
BindingDB Entry DOI: 10.7270/Q2M61PKJ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50455530
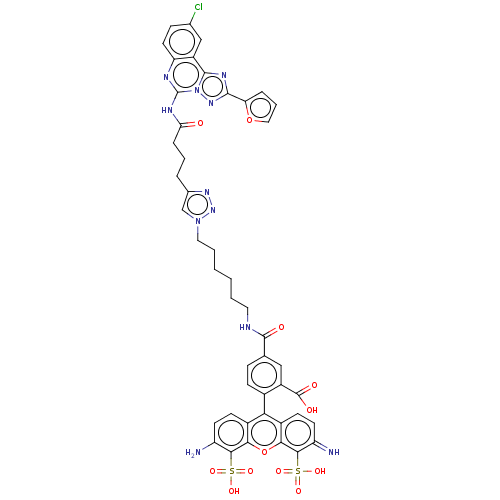
(CHEMBL2413248)Show SMILES Nc1ccc2c(-c3ccc(cc3C(O)=O)C(=O)NCCCCCCn3cc(CCCC(=O)Nc4nc5ccc(Cl)cc5c5nc(nn45)-c4ccco4)nn3)c3ccc(=N)c(c3oc2c1S(O)(=O)=O)S(O)(=O)=O |(2.4,-1.39,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-2.67,1.54,;-2.67,3.08,;-4,3.85,;-4,5.39,;-2.67,6.16,;-1.34,5.39,;-1.33,3.85,;0,3.09,;.01,1.86,;1.07,3.71,;-2.67,7.7,;-3.74,8.32,;-1.34,8.47,;-1.34,10.01,;-0,10.78,;-0,12.32,;1.33,13.1,;1.33,14.64,;2.67,15.41,;2.67,16.95,;3.91,17.83,;3.43,19.3,;4.33,20.54,;3.7,21.95,;4.6,23.2,;3.97,24.61,;2.75,24.73,;4.87,25.86,;4.24,27.26,;2.71,27.41,;2.08,28.82,;.55,28.97,;-.09,30.37,;.81,31.63,;.3,32.75,;2.34,31.47,;2.98,30.07,;4.51,29.92,;5.65,30.95,;6.98,30.19,;6.67,28.68,;5.14,28.51,;8.39,30.82,;9.64,29.94,;10.86,30.89,;10.33,32.34,;8.8,32.29,;1.89,19.29,;1.42,17.83,;-4,.77,;-5.33,1.54,;-6.67,.77,;-6.67,-.77,;-7.74,-1.39,;-5.33,-1.54,;-4,-.77,;-2.67,-1.54,;-1.33,-.77,;,-1.54,;,-3.08,;0,-4.31,;-1.07,-3.7,;1.07,-3.7,;-5.33,-3.08,;-5.33,-4.31,;-6.4,-3.7,;-4.27,-3.7,)| Show InChI InChI=1S/C46H40ClN11O12S2/c47-25-11-17-34-31(22-25)43-53-42(35-8-6-20-69-35)55-58(43)46(51-34)52-36(59)9-5-7-26-23-57(56-54-26)19-4-2-1-3-18-50-44(60)24-10-12-27(30(21-24)45(61)62)37-28-13-15-32(48)40(71(63,64)65)38(28)70-39-29(37)14-16-33(49)41(39)72(66,67)68/h6,8,10-17,20-23,48H,1-5,7,9,18-19,49H2,(H,50,60)(H,61,62)(H,51,52,59)(H,63,64,65)(H,66,67,68) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from recombinant human adenosine A3 receptor expressed in CHO cell membranes after 1 hr by gamma counting method |
J Med Chem 61: 4301-4316 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01627
BindingDB Entry DOI: 10.7270/Q23R0WG5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data