

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enoyl-[acyl-carrier-protein] reductase [NADPH] FabI [9-251] (Staphylococcus aureus) | BDBM97445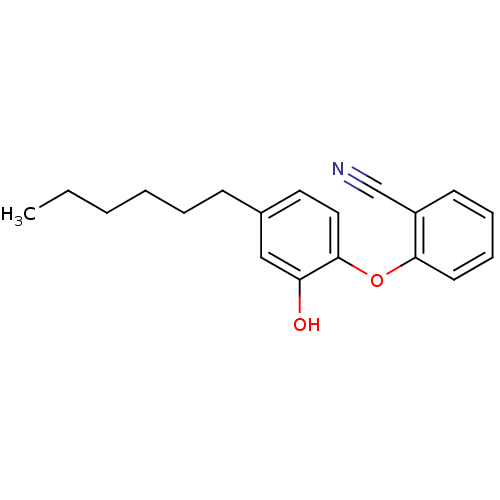 (PT119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stony Brook University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus enoyl ACP reductase | Eur J Med Chem 88: 66-73 (2014) Article DOI: 10.1016/j.ejmech.2014.09.008 BindingDB Entry DOI: 10.7270/Q25T3N3S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069294 ((S)-3-[4-(carbohydrazonamidol)-phenyl]-N-cyclopent...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426499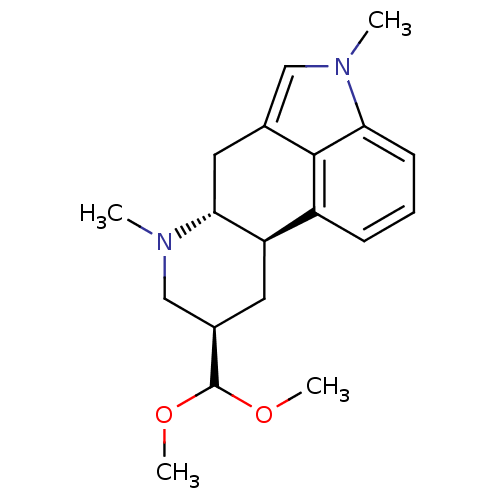 (CHEMBL2323581) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426500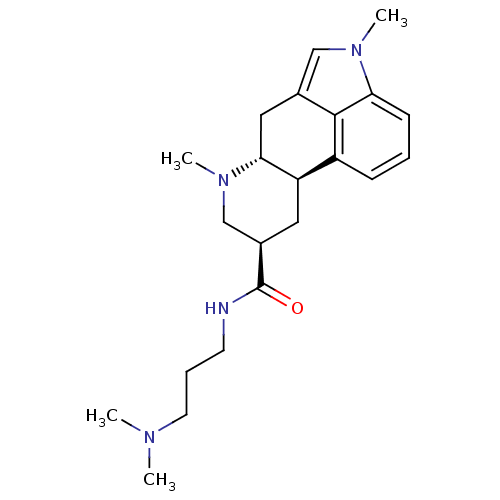 (CHEMBL2323580) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426498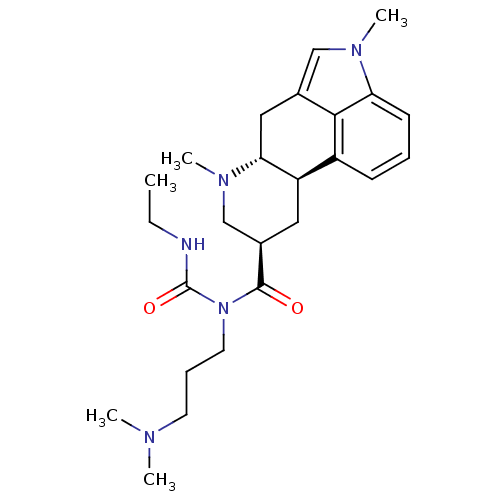 (1,6-Dimethylcabergoline | CHEMBL2323579) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426497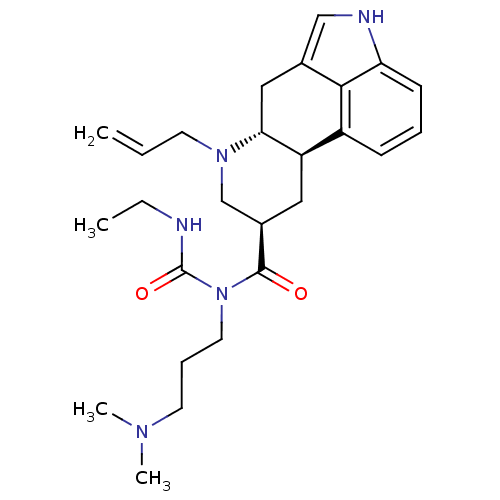 (CABERGOLINE | Dostinex | FCE-21336) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50070181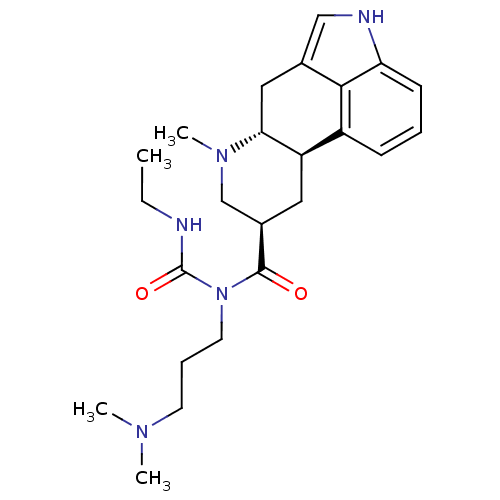 (1-(3-Dimethylamino-propyl)-3-ethyl-1-((6aR,9R,10aR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071729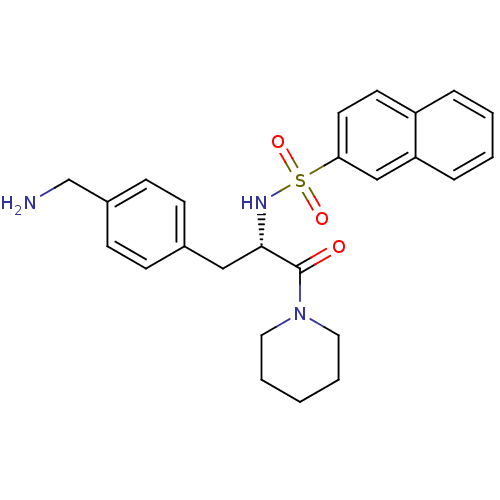 (CHEMBL313826 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071723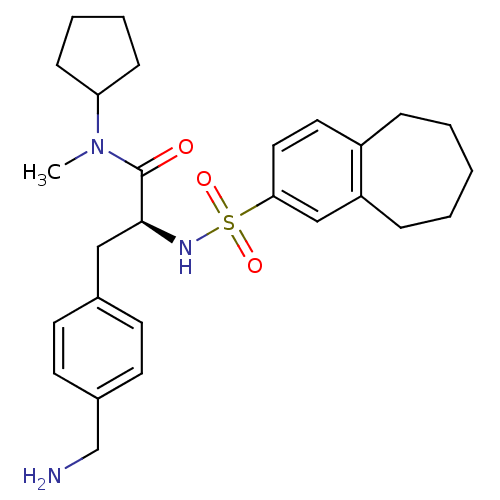 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071725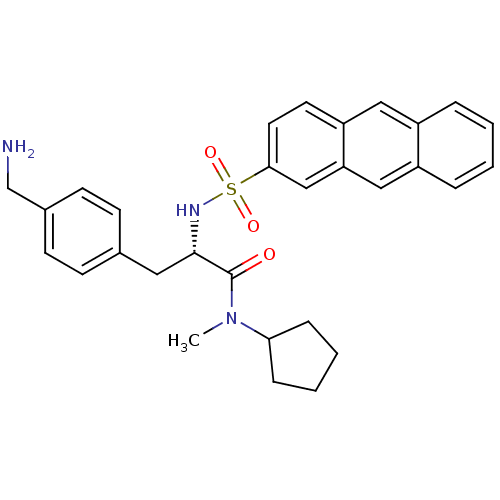 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071722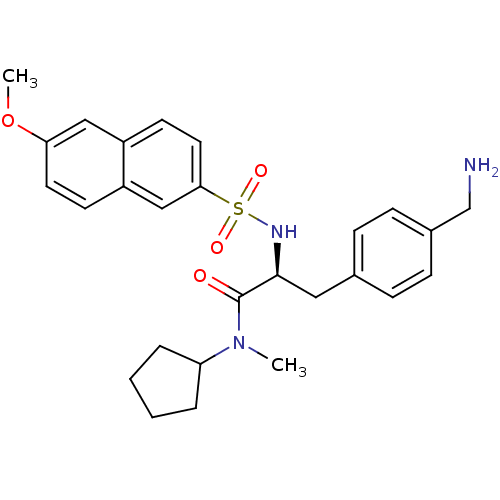 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070783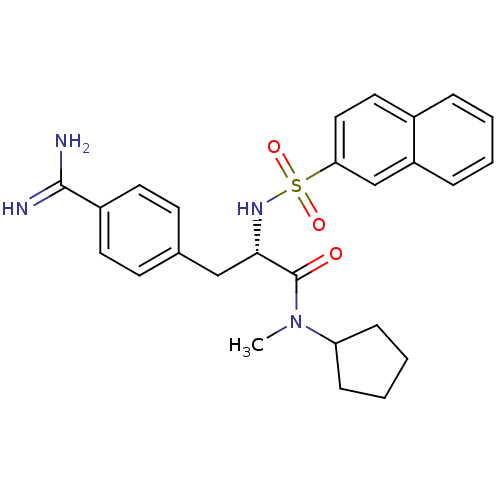 ((S)-3-(4-Carbamimidoyl-phenyl)-N-cyclopentyl-N-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50426501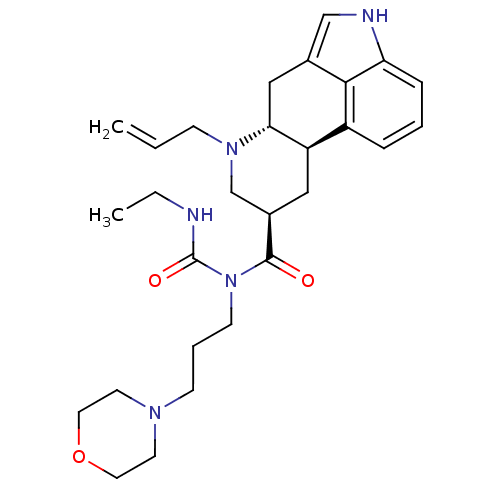 (CHEMBL2323578) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Displacement of [125I](+/-)DOI from human 5-HT2B receptor trasfected in CHO cell membrane after 60 mins by scintillation counting analysis | ACS Med Chem Lett 4: 254-258 (2013) Article DOI: 10.1021/ml3003814 BindingDB Entry DOI: 10.7270/Q2S183T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50401014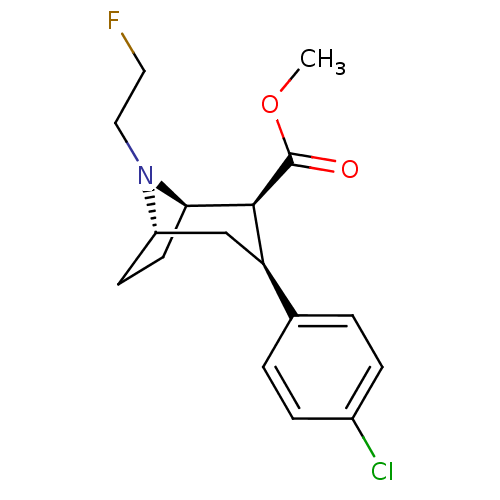 (CHEMBL2206307) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johannes Gutenberg University Curated by ChEMBL | Assay Description Binding affinity to DAT | Bioorg Med Chem Lett 22: 679-82 (2011) Article DOI: 10.1016/j.bmcl.2011.10.053 BindingDB Entry DOI: 10.7270/Q2ZW1N2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053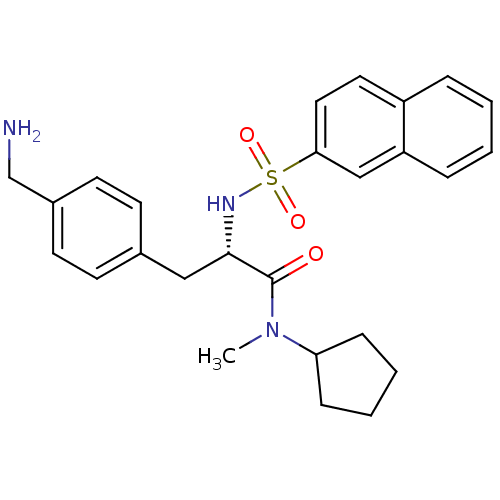 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071721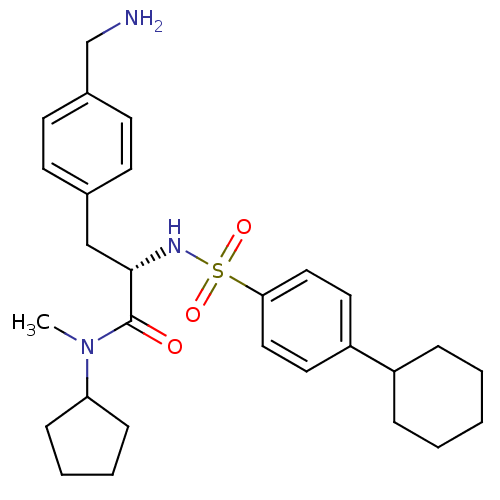 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069053 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071728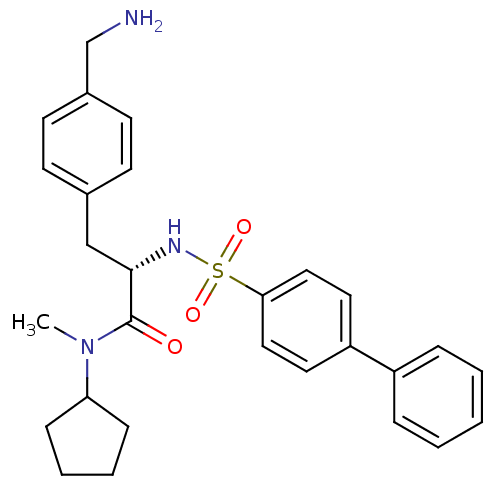 ((S)-3-(4-Aminomethyl-phenyl)-2-(biphenyl-4-sulfony...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50071727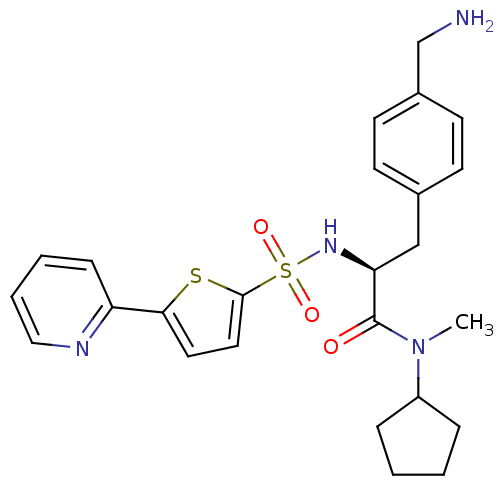 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human thrombin | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069052 (CHEMBL164661 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069051 ((S)-3-(4-Aminomethyl-phenyl)-N-isopropyl-N-methyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069057 ((S)-3-(4-Aminomethyl-phenyl)-N,N-dimethyl-2-(napht...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071725 ((S)-3-(4-Aminomethyl-phenyl)-2-(anthracene-2-sulfo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071721 ((S)-3-(4-Aminomethyl-phenyl)-2-(4-cyclohexyl-benze...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071723 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50069055 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071726 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50069053 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-N-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071722 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069056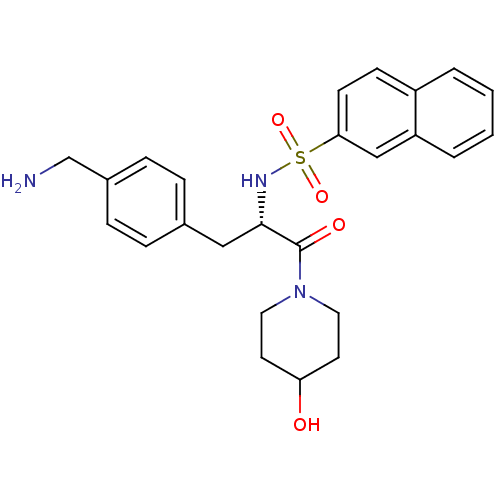 (CHEMBL350751 | Naphthalene-2-sulfonic acid [(S)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50071724 ((S)-3-(4-Aminomethyl-phenyl)-N-cyclopentyl-2-(5-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Binding affinity of the compound towards bovine trypsin was evaluated in vitro | Bioorg Med Chem Lett 8: 2563-8 (1999) BindingDB Entry DOI: 10.7270/Q20Z72FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069054 ((S)-3-(4-Aminomethyl-phenyl)-N-isopropyl-2-(naphth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069050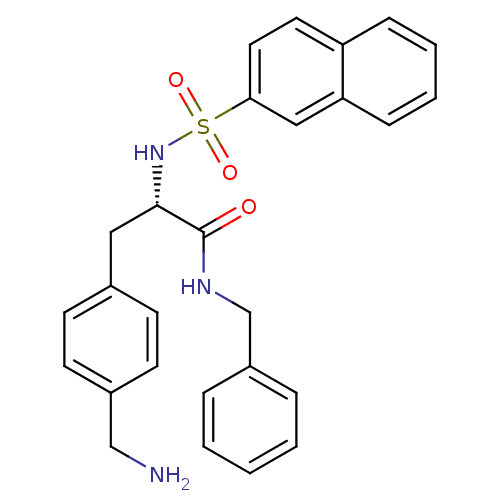 ((S)-3-(4-Aminomethyl-phenyl)-N-benzyl-2-(naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biotech Research Institute Curated by ChEMBL | Assay Description Inhibition constant was evaluated for inhibitory activity against the blood coagulant thrombin | Bioorg Med Chem Lett 8: 735-8 (1999) BindingDB Entry DOI: 10.7270/Q2668C95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138211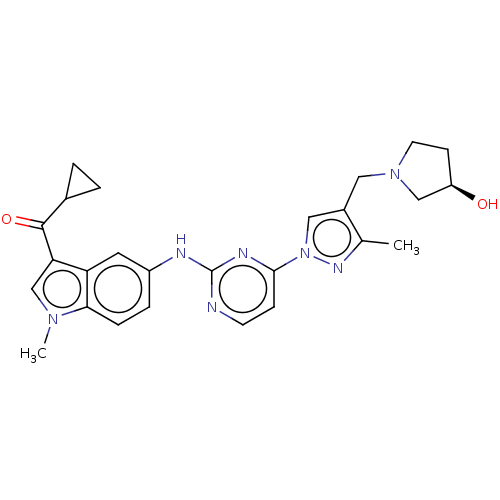 (US8871778, 190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138212 (US8871778, 198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138217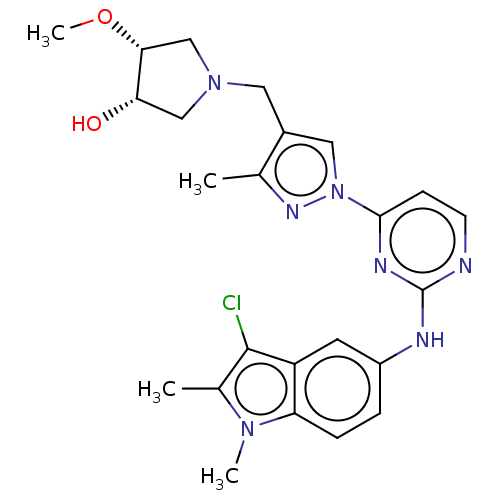 (US8871778, 251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304788 (CHEMBL594160 | methyl 4-(3-chloro-4-methoxybenzyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138204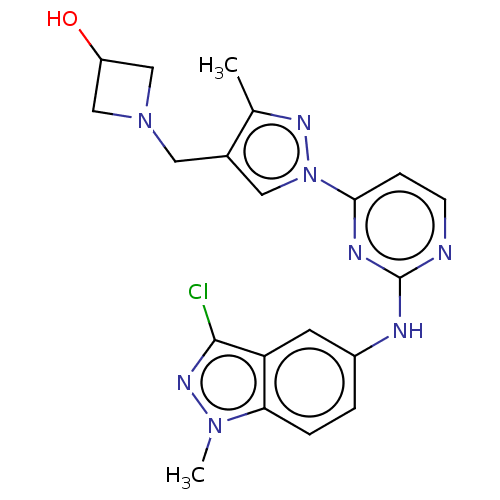 (US8871778, 161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Kangwon National University Curated by ChEMBL | Assay Description Inhibition of purified full-length human SYK pre-incubated for 30 mins at room temperature before Ulight-TK peptide substrate addition and measured 1... | Bioorg Med Chem Lett 25: 4441-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.011 BindingDB Entry DOI: 10.7270/Q22Z17BN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138219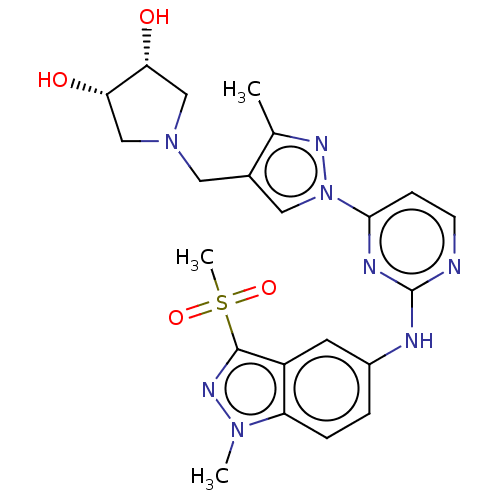 (US8871778, 255) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138201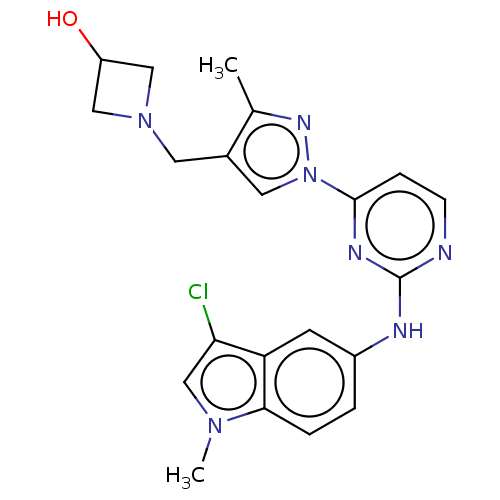 (US8871778, 144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Kangwon National University Curated by ChEMBL | Assay Description Inhibition of purified full-length human SYK pre-incubated for 30 mins at room temperature before Ulight-TK peptide substrate addition and measured 1... | Bioorg Med Chem Lett 25: 4441-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.011 BindingDB Entry DOI: 10.7270/Q22Z17BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138210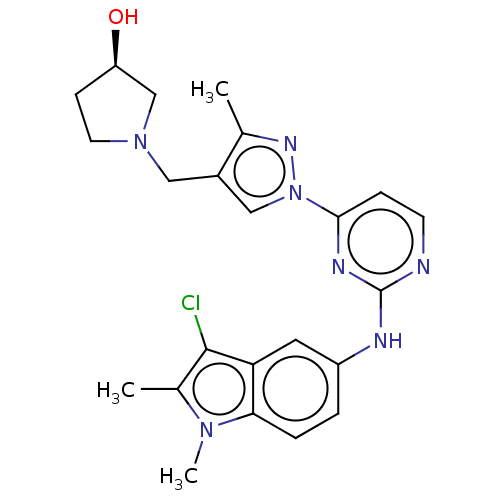 (US8871778, 189) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Kangwon National University Curated by ChEMBL | Assay Description Inhibition of purified full-length human SYK pre-incubated for 30 mins at room temperature before Ulight-TK peptide substrate addition and measured 1... | Bioorg Med Chem Lett 25: 4441-6 (2015) Article DOI: 10.1016/j.bmcl.2015.09.011 BindingDB Entry DOI: 10.7270/Q22Z17BN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50246252 (CHEMBL521075 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138200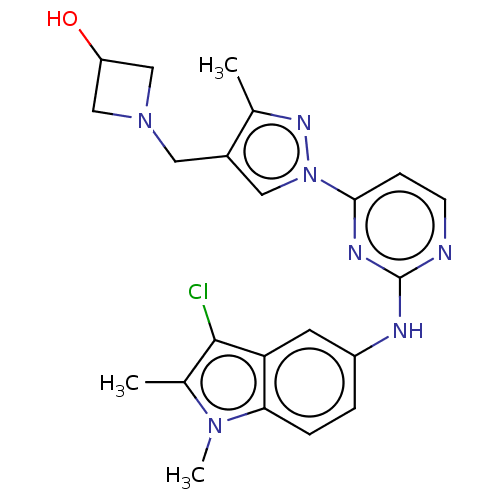 (US8871778, 141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304789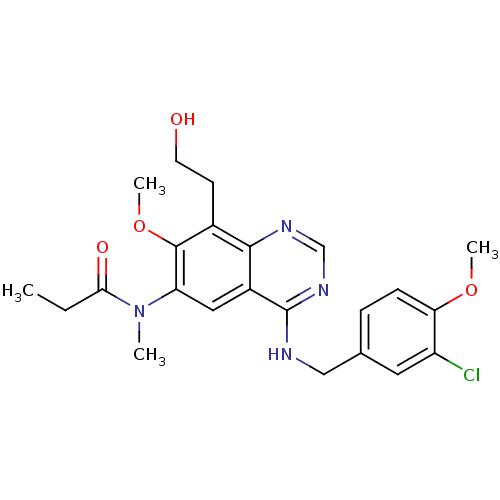 (CHEMBL604271 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK (Homo sapiens (Human)) | BDBM138207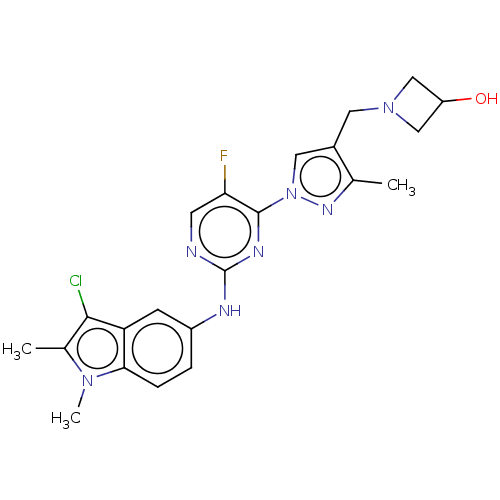 (US8871778, 176) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Genosco; Oscotec, Inc. US Patent | Assay Description Compounds of the invention were initially diluted to 10 mM in 100% DMSO (CALBIOCHEM) for storage and made into kinase buffer solution to create a com... | US Patent US8871778 (2014) BindingDB Entry DOI: 10.7270/Q2X065R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Bos taurus) | BDBM50304792 (CHEMBL595104 | N-(4-(3-chloro-4-methoxybenzylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Research Institute Curated by ChEMBL | Assay Description Inhibition of bovine platelet PDE5 | Bioorg Med Chem Lett 20: 383-6 (2010) Article DOI: 10.1016/j.bmcl.2009.10.071 BindingDB Entry DOI: 10.7270/Q28P60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1247 total ) | Next | Last >> |