

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Menin (Homo sapiens (Human)) | BDBM50582737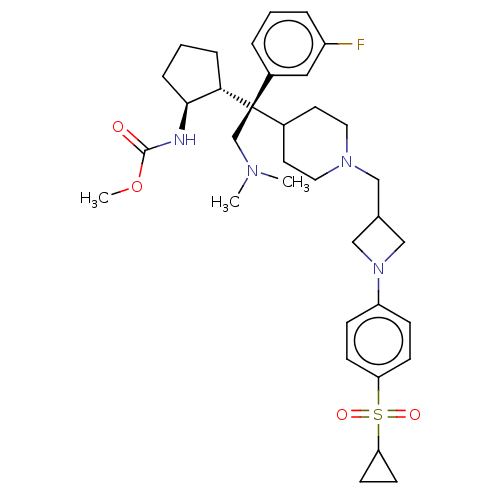 (CHEMBL5076146) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxopain-2 (Toxoplasma gondii) | BDBM50271690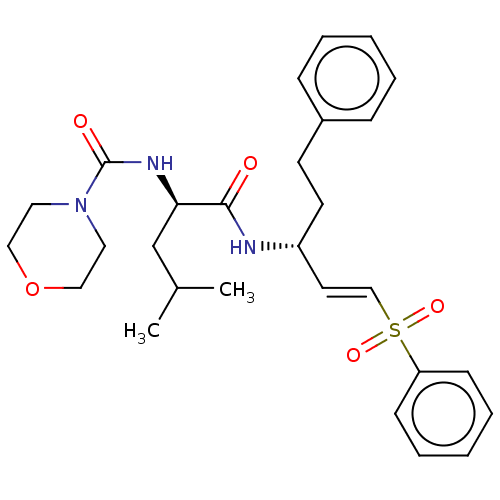 (CHEMBL222649) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins ... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582744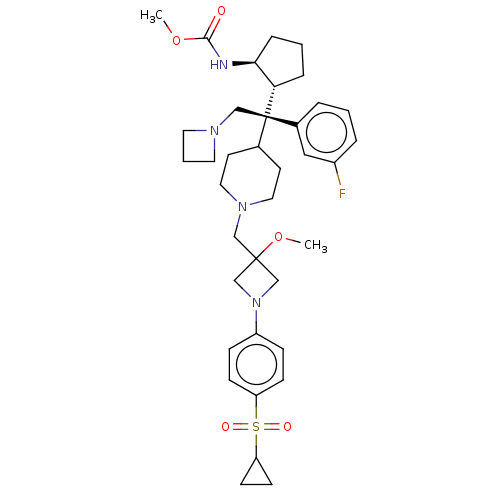 (CHEMBL5085940) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582751 (CHEMBL5094183) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582746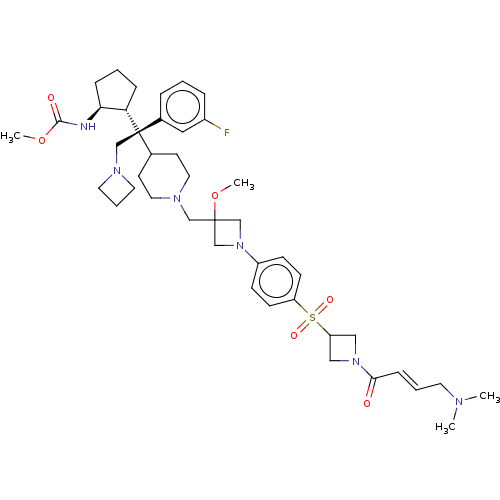 (CHEMBL5081367) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582738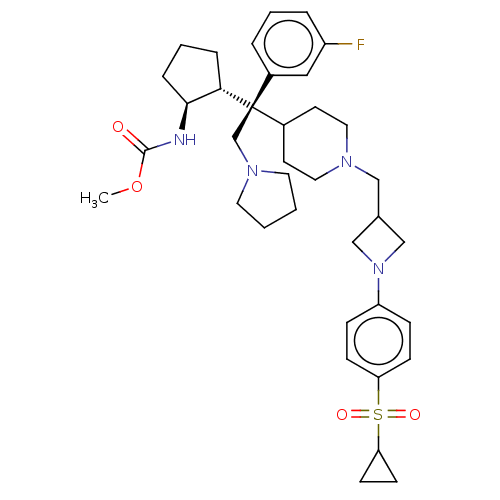 (CHEMBL5071412) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582747 (CHEMBL5079678) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582735 (CHEMBL5090509) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582749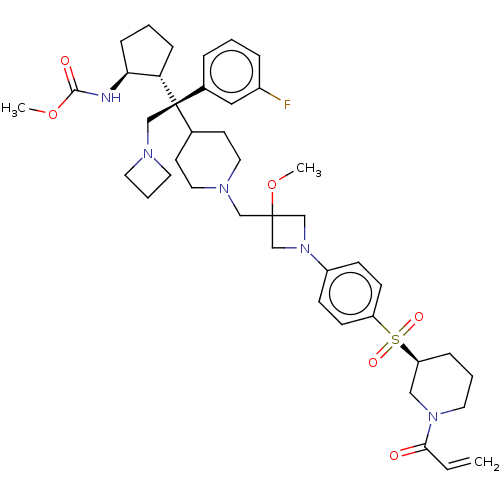 (CHEMBL5079839) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271690 (CHEMBL222649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582734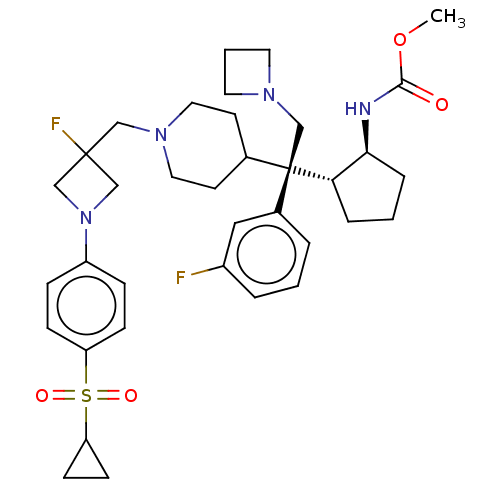 (CHEMBL5088651) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582740 (CHEMBL5088354) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582739 (CHEMBL5082413) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582736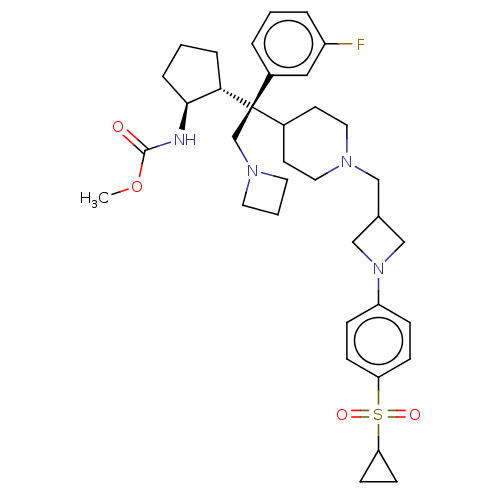 (CHEMBL5075855) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582743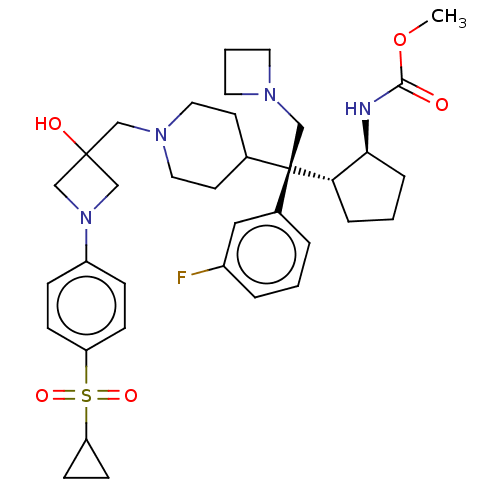 (CHEMBL5086858) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582750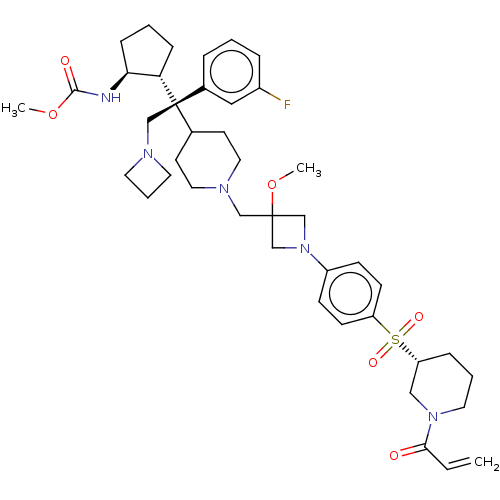 (CHEMBL5083305) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582748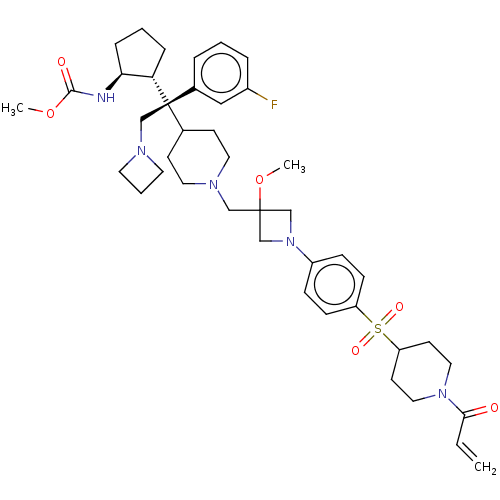 (CHEMBL5081374) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582741 (CHEMBL5087485) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582753 (CHEMBL5081163) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582752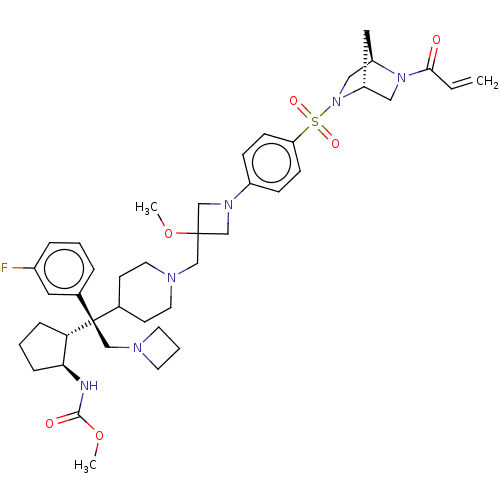 (CHEMBL5085064) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582742 (CHEMBL5076001) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173310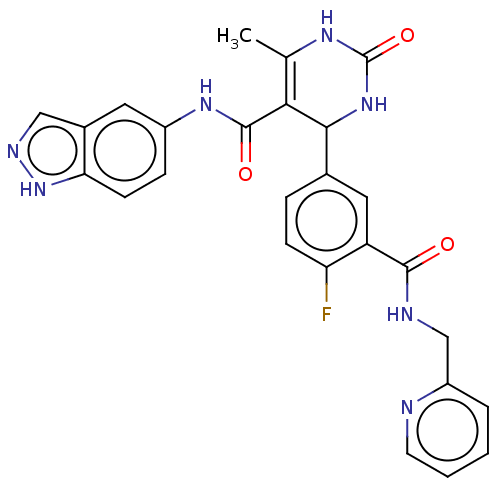 (CHEMBL3808660 | US10023564, Example 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173307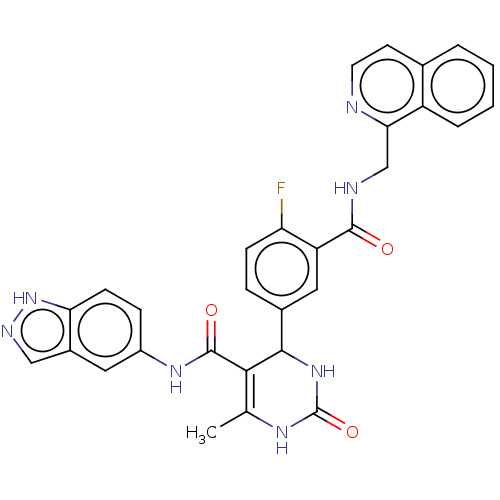 (CHEMBL3809796 | US10023564, Example 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355756 (CHEMBL1909658) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271681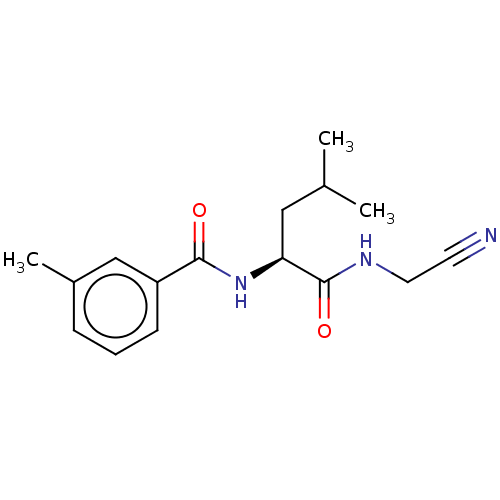 (CHEMBL4129611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355755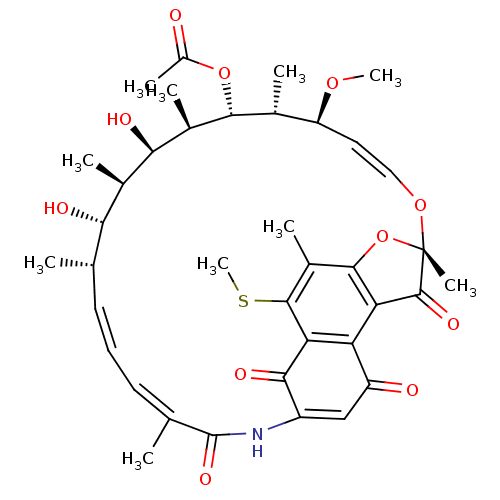 (CHEMBL1911296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173315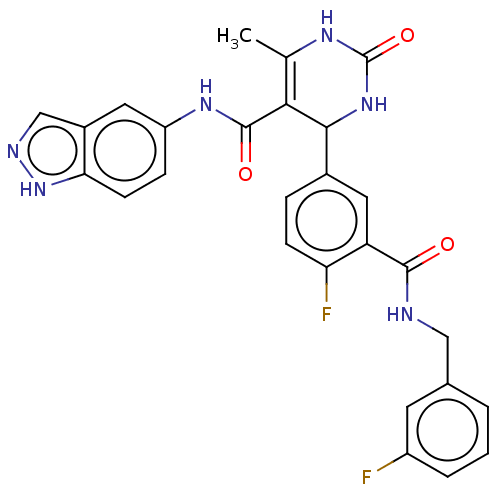 (CHEMBL3809100 | US10023564, Example 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA-directed RNA polymerase subunit beta (Mycobacterium tuberculosis) | BDBM50355751 (CHEMBL1911286) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of wild type Mycobacterium tuberculosis RNA polymerase after 10 mins using ribo green staining by rolling circle transcription assay | Bioorg Med Chem Lett 21: 6094-9 (2011) Article DOI: 10.1016/j.bmcl.2011.08.054 BindingDB Entry DOI: 10.7270/Q2R78FMB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173305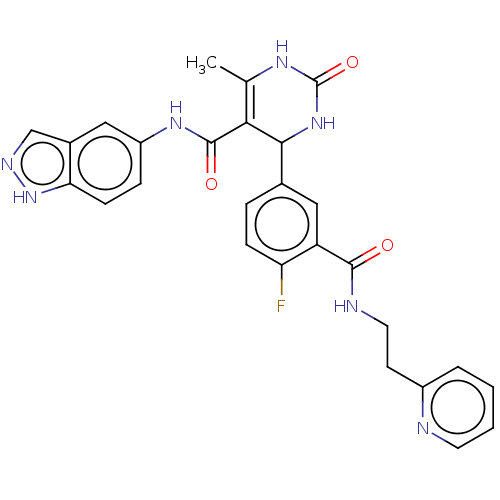 (CHEMBL3810107 | US10023564, Example 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Menin (Homo sapiens (Human)) | BDBM50582745 (CHEMBL5084863) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to menin (unknown origin) incubated for 1 hr by fluorescence polarization-based competitive binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00789 BindingDB Entry DOI: 10.7270/Q2GX4GFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271692 (CHEMBL4126611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM31993 (Dipeptidyl nitrile inhibitor, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271697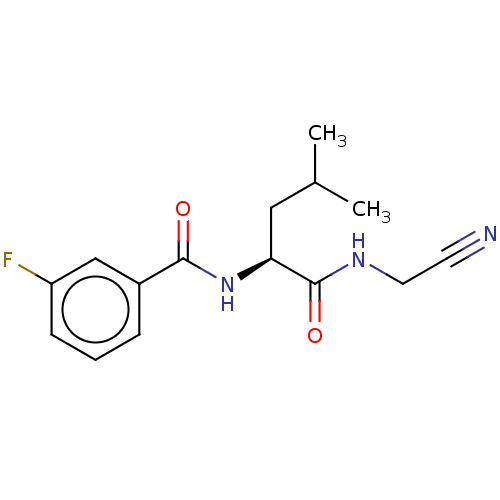 (CHEMBL4128244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173311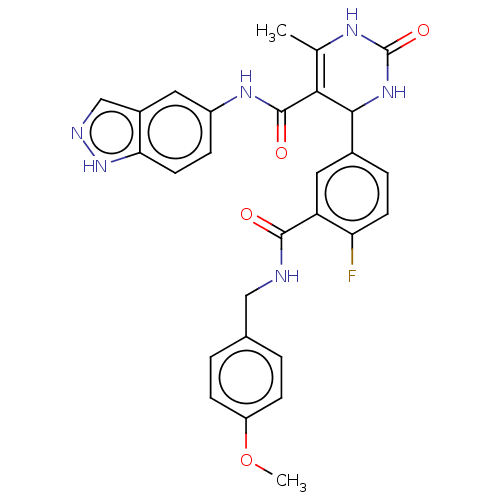 (CHEMBL3810073 | US10023564, Example 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173325 (CHEMBL3809965 | US10023564, Example 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173314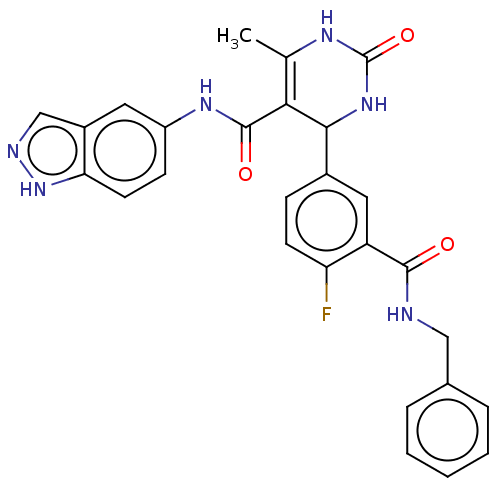 (CHEMBL3808840 | US10023564, Example 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271696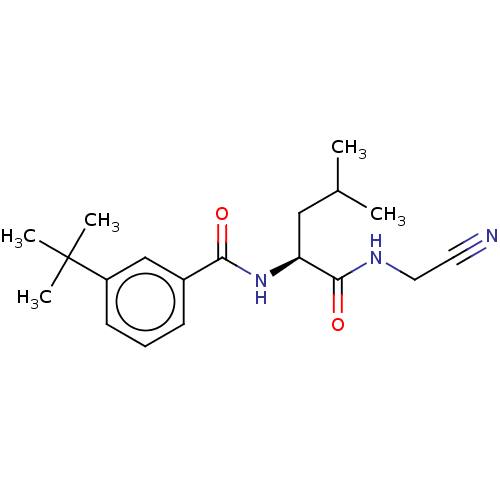 (CHEMBL4125776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271685 (CHEMBL4128887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173306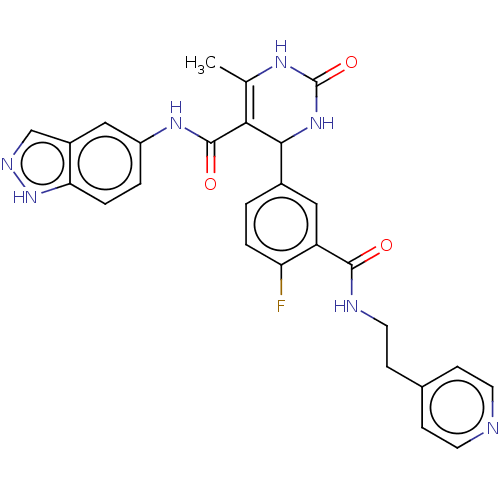 (CHEMBL3808565 | US10023564, Example 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271694 (CHEMBL4127007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271693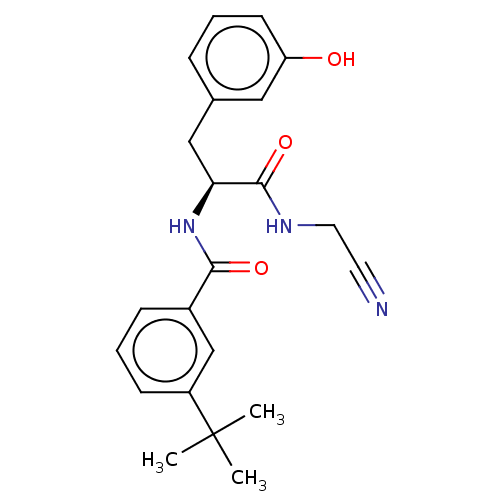 (CHEMBL4126455) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271714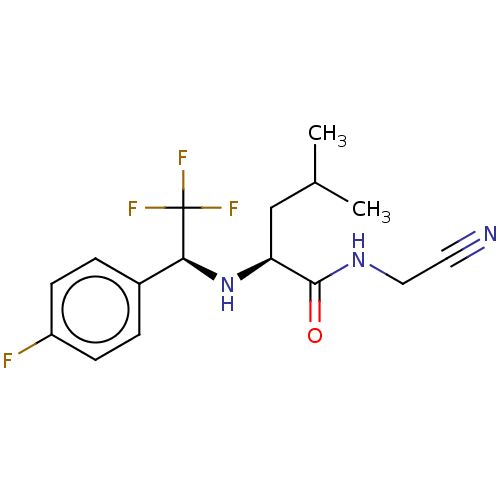 (CHEMBL4126546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173312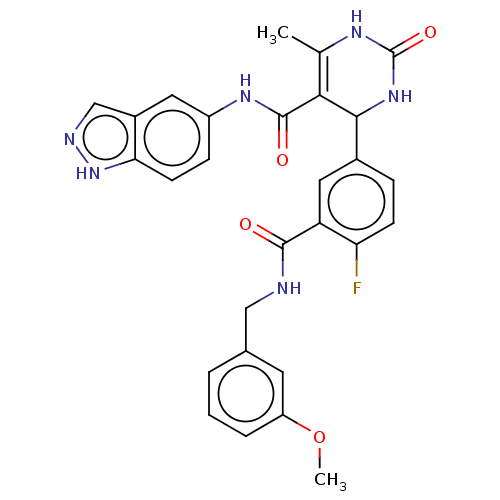 (CHEMBL3810312 | US10023564, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rhodopsin kinase GRK1 (Homo sapiens (Human)) | BDBM50173307 (CHEMBL3809796 | US10023564, Example 8) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of GRK1 (unknown origin) using tubulin as substrate by SDS-PAGE method | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173320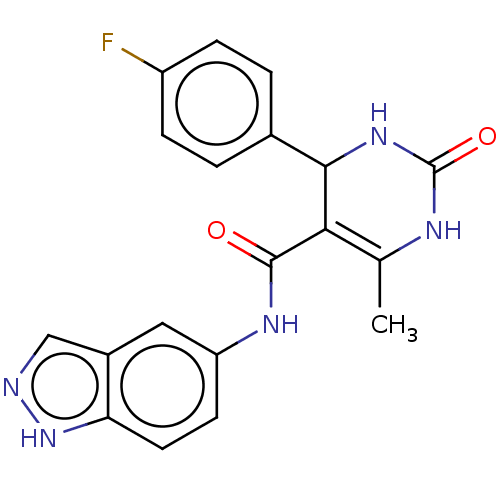 (GSK-180736A | GSK180736A | US10023564, Compound GS...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19861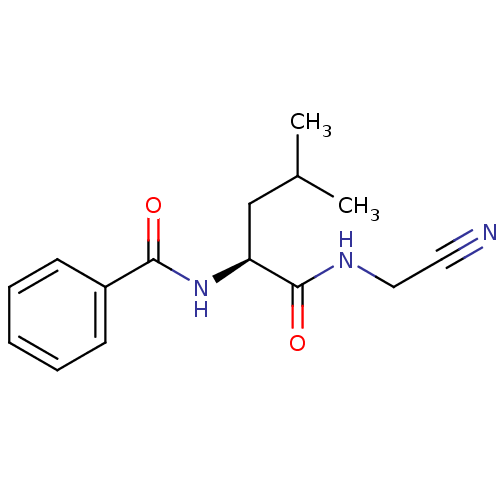 ((2S)-N-(cyanomethyl)-4-methyl-2-(phenylformamido)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50173323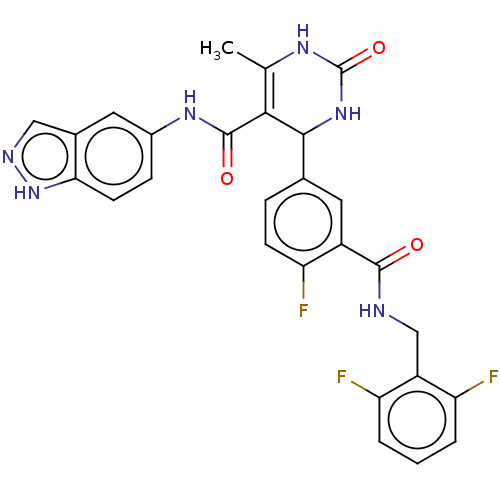 (CHEMBL3808621 | US10023564, Example 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Temple University Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) by ADP-Glo kinase assay | J Med Chem 59: 3793-807 (2016) Article DOI: 10.1021/acs.jmedchem.5b02000 BindingDB Entry DOI: 10.7270/Q2JQ12X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50271689 (CHEMBL4130028) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of human cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins by spectroph... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxopain-2 (Toxoplasma gondii) | BDBM50271714 (CHEMBL4126546) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins ... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Toxopain-2 (Toxoplasma gondii) | BDBM50271696 (CHEMBL4125776) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Curated by ChEMBL | Assay Description Inhibition of Toxoplasma gondii cathepsin L using Z-L-R-AMC as substrate preincubated for 5 mins followed by substrate addition measured over 5 mins ... | Bioorg Med Chem Lett 28: 1972-1980 (2018) Article DOI: 10.1016/j.bmcl.2018.03.020 BindingDB Entry DOI: 10.7270/Q2445PZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 333 total ) | Next | Last >> |