

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity at human P2Y14R expressed in CHO cells assessed as inhibition of UDPG-mediated reduction of forskolin-induced [3H]cAMP production... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50532693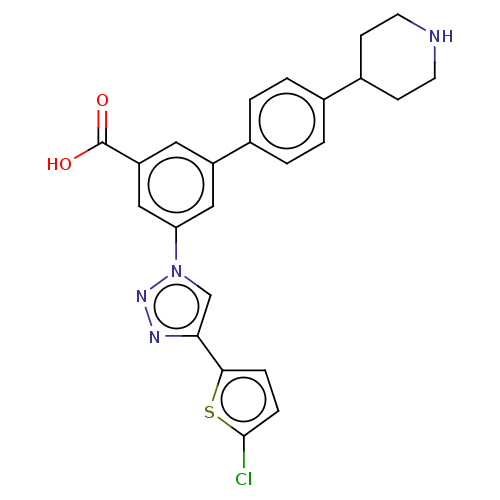 (CHEMBL4544251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human H1 histamine receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2C receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50532691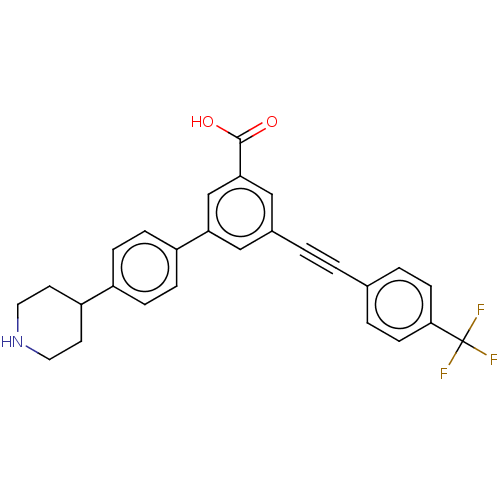 (CHEMBL4455037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to guinea pig sigma1 receptor by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50532691 (CHEMBL4455037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to guinea pig sigma1 receptor by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50532693 (CHEMBL4544251) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human alpha2A receptor expressed in MDCK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to human delta opioid receptor expressed in HEK cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50532691 (CHEMBL4455037) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma intracellular receptor 2 (Rattus norvegicus (Rat)) | BDBM50532691 (CHEMBL4455037) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to sigma 2 receptor in rat PC3 cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50456168 (CHEMBL1800685) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50456168 (CHEMBL1800685) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 6.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity to rat D3 dopamine receptor expressed in HEK293T cells by PDSP assay | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Antagonist activity human P2Y14R expressed in African green monkey COS7 cells assessed as inhibition of UDPG-induced [3H]inositol phosphate accumulat... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521692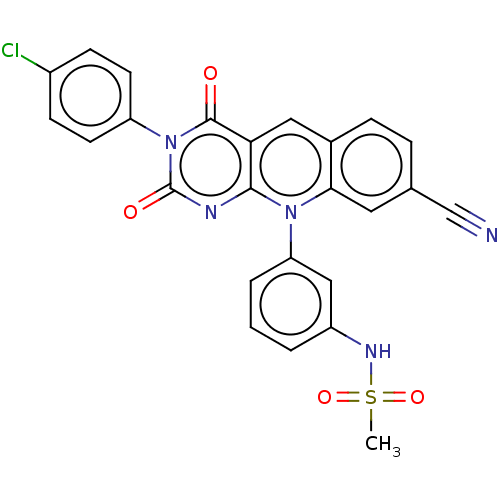 (CHEMBL4441013) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521699 (CHEMBL4572376) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521689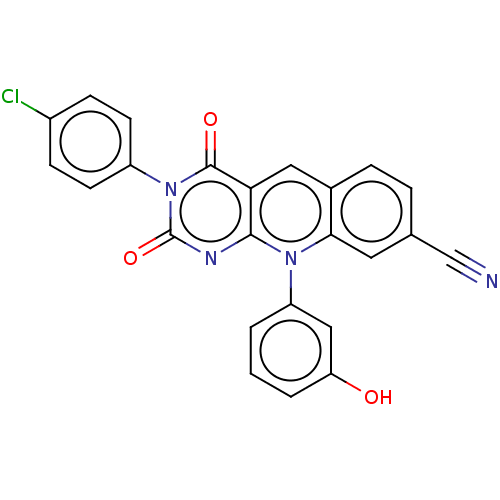 (CHEMBL4436202) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521691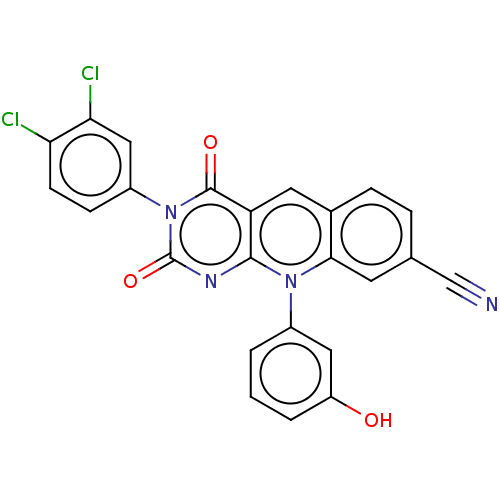 (CHEMBL4587441) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521690 (CHEMBL4520052) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM188529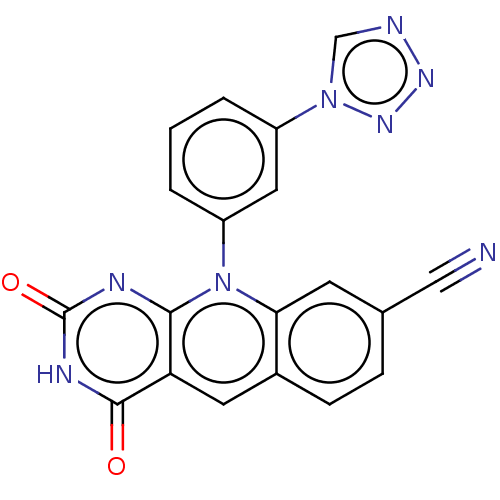 (2,4-Dioxo-10-[3-(1H-tetrazol-5-yl)phenyl]pyrimido[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National Institutes of Health | Assay Description Ten-million cells (1 x 107), either human, chicken DT40 wild type, or knockout for TDP2 and complemented with human TDP2, were collected, washed, and... | ACS Chem Biol 11: 1925-33 (2016) Article DOI: 10.1021/acschembio.5b01047 BindingDB Entry DOI: 10.7270/Q2G15ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 [E242G,S244A,Q278R,I281T,K282R,R316G,P318T,Y321CH323L] (Mus musculus (Mouse)) | BDBM188529 (2,4-Dioxo-10-[3-(1H-tetrazol-5-yl)phenyl]pyrimido[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National Institutes of Health | Assay Description TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&... | ACS Chem Biol 11: 1925-33 (2016) Article DOI: 10.1021/acschembio.5b01047 BindingDB Entry DOI: 10.7270/Q2G15ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532694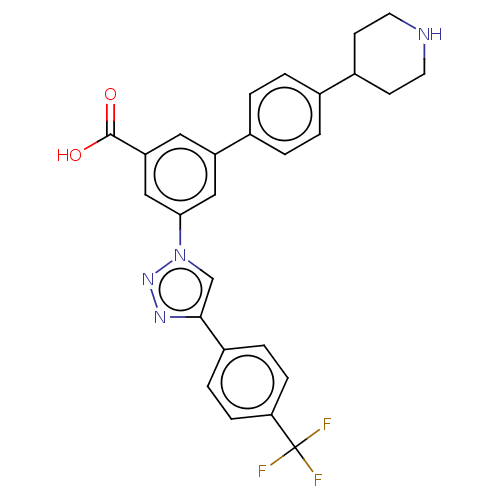 (CHEMBL4454538) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532694 (CHEMBL4454538) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM188529 (2,4-Dioxo-10-[3-(1H-tetrazol-5-yl)phenyl]pyrimido[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National Institutes of Health | Assay Description Ten-million cells (1 x 107), either human, chicken DT40 wild type, or knockout for TDP2 and complemented with human TDP2, were collected, washed, and... | ACS Chem Biol 11: 1925-33 (2016) Article DOI: 10.1021/acschembio.5b01047 BindingDB Entry DOI: 10.7270/Q2G15ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521687 (CHEMBL4560735) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521697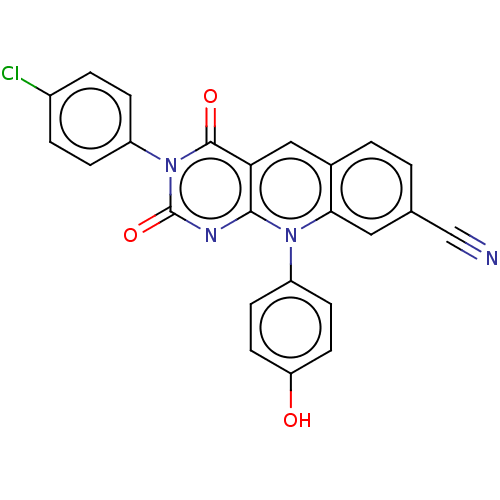 (CHEMBL4468174) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521698 (CHEMBL4555612) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521678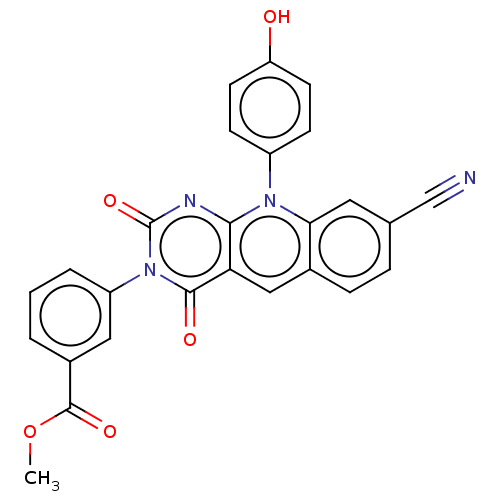 (CHEMBL4521151) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM188529 (2,4-Dioxo-10-[3-(1H-tetrazol-5-yl)phenyl]pyrimido[...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National Institutes of Health | Assay Description TDP2 reactions were carried out as described previously23 with the following modifications. A 18-mer single-stranded oligonucleotide DNA substrate (&... | ACS Chem Biol 11: 1925-33 (2016) Article DOI: 10.1021/acschembio.5b01047 BindingDB Entry DOI: 10.7270/Q2G15ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438848 (CHEMBL2420480) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438864 (CHEMBL2420507) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438863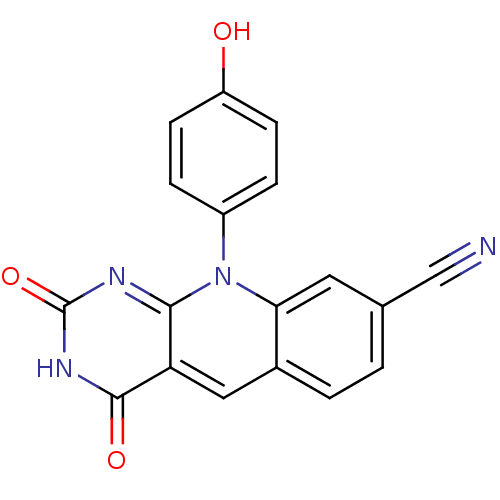 (10-(4-Hydroxyphenyl)-2,4-dioxo-pyrimido[4,5-b]quin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | n/a |
National Institutes of Health | Assay Description Ten-million cells (1 x 107), either human, chicken DT40 wild type, or knockout for TDP2 and complemented with human TDP2, were collected, washed, and... | ACS Chem Biol 11: 1925-33 (2016) Article DOI: 10.1021/acschembio.5b01047 BindingDB Entry DOI: 10.7270/Q2G15ZN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521701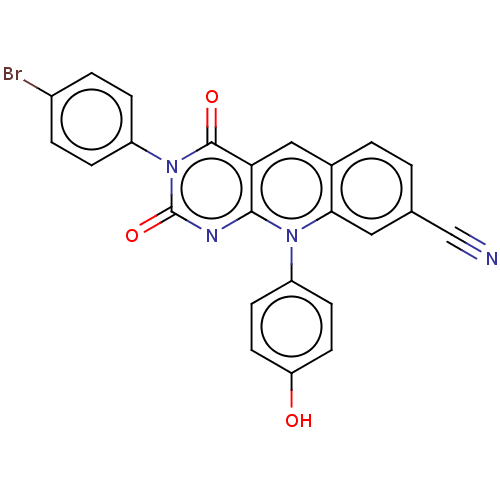 (CHEMBL4457155) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521688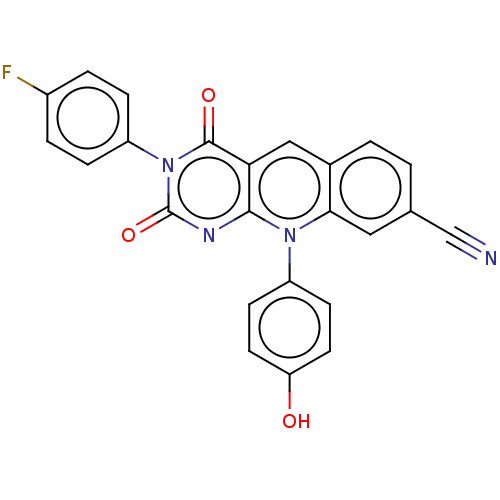 (CHEMBL4546104) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532697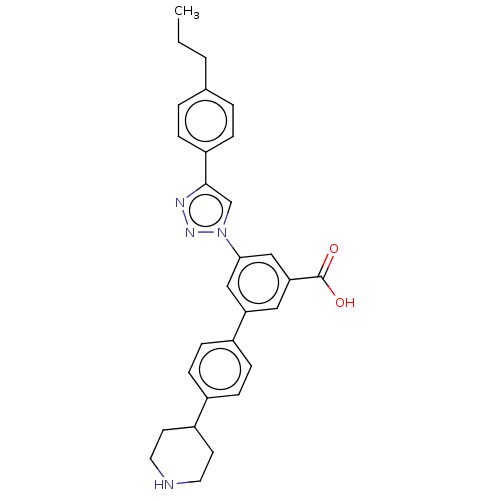 (CHEMBL4441075) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532697 (CHEMBL4441075) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532701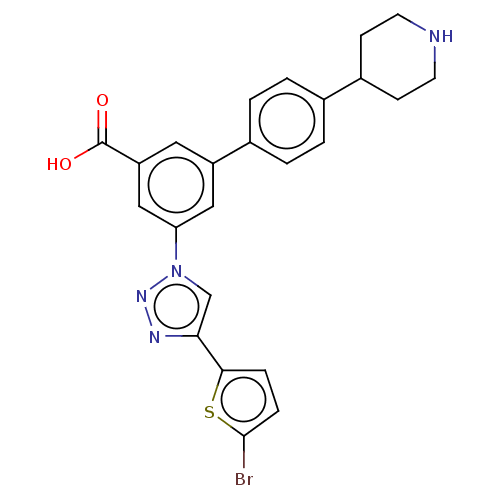 (CHEMBL4472517) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532701 (CHEMBL4472517) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532696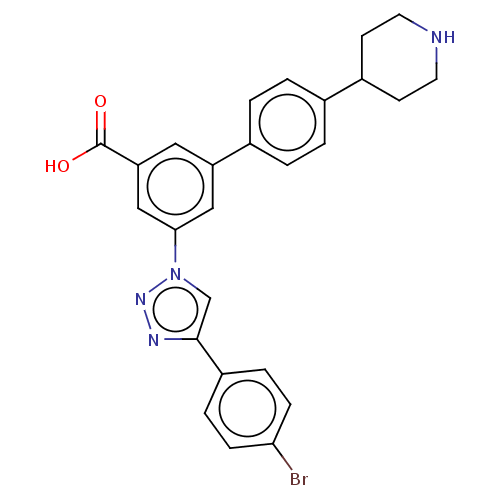 (CHEMBL4465763) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532696 (CHEMBL4465763) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50521702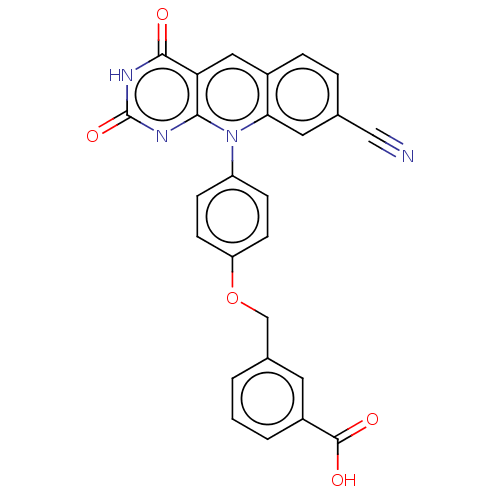 (CHEMBL4518508) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532702 (CHEMBL4458050) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50532702 (CHEMBL4458050) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of 6-Amino-9-(2-carboxy-4-((6-(4-(4-(4-(4-(3-carboxy-6-(4-(trifluoromethyl)phenyl)-naphthalen-1-yl)phenyl)piperidin-1-yl)-butyl)-1H-1,2,... | J Med Chem 59: 6149-68 (2016) Article DOI: 10.1021/acs.jmedchem.6b00044 BindingDB Entry DOI: 10.7270/Q2DJ5K34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosyl-DNA phosphodiesterase 2 (Homo sapiens (Human)) | BDBM50438863 (10-(4-Hydroxyphenyl)-2,4-dioxo-pyrimido[4,5-b]quin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of humanized zebrafish TDP2 using 5'-(6-FAM-NHS) as substrate preincubated for 10 mins followed by substrate addition and measured after 6... | J Med Chem 62: 4669-4682 (2019) Article DOI: 10.1021/acs.jmedchem.9b00274 BindingDB Entry DOI: 10.7270/Q2F1934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |