

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477148 (CHEMBL235870) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477143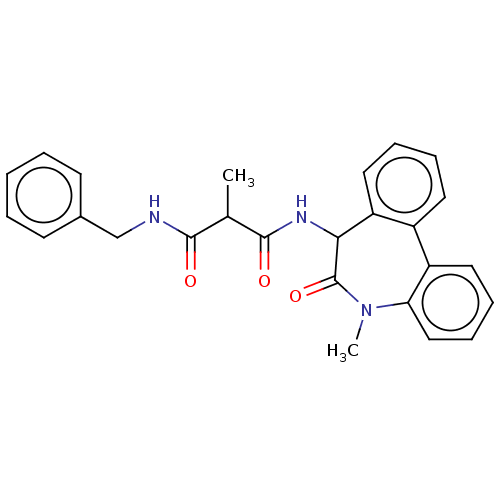 (CHEMBL392113) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477136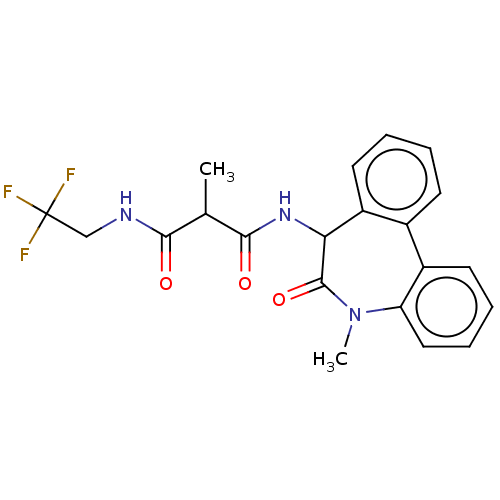 (CHEMBL235659) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477131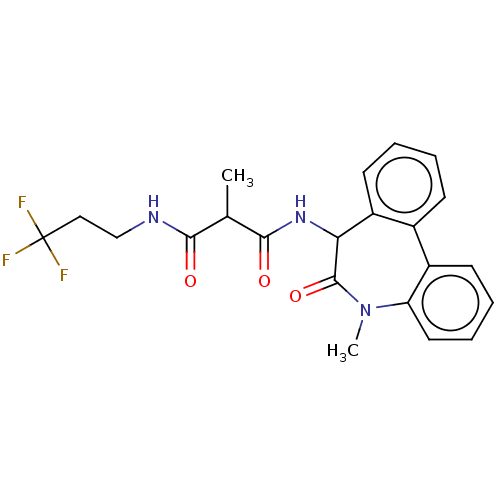 (CHEMBL235869) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477131 (CHEMBL235869) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477136 (CHEMBL235659) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477143 (CHEMBL392113) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477134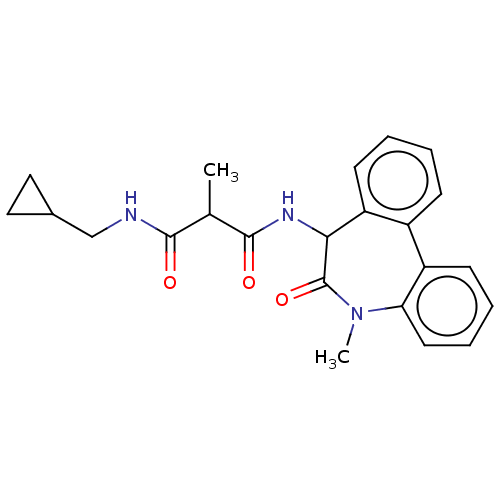 (CHEMBL393542) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477134 (CHEMBL393542) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477145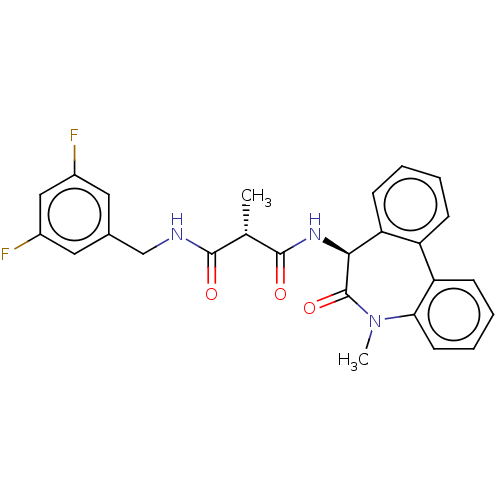 (CHEMBL235648) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477146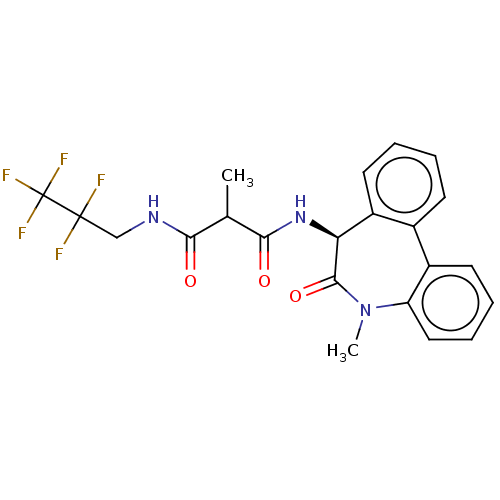 (CHEMBL428374) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477142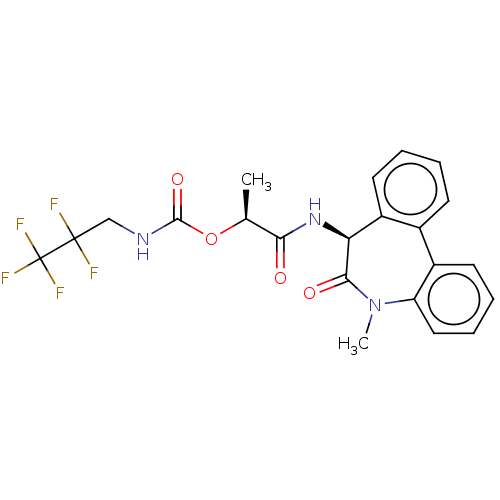 (CHEMBL235226) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273103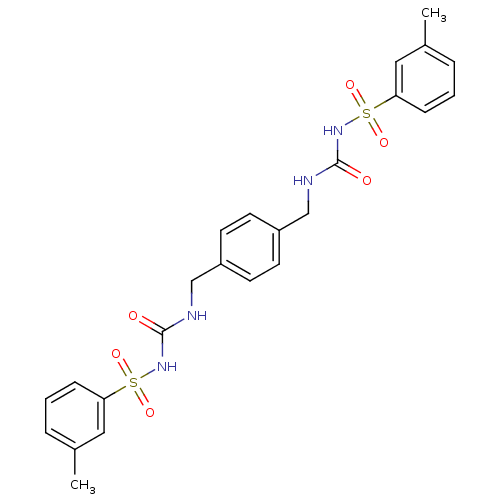 (3-[(3-methylbenzene)sulfonyl]-1-({4-[({[(3-methylb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415679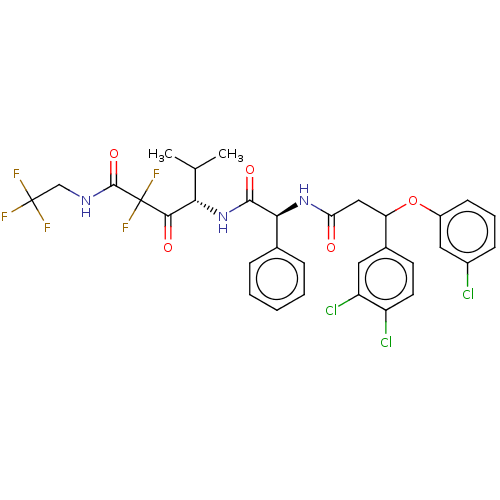 (US10428108, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415662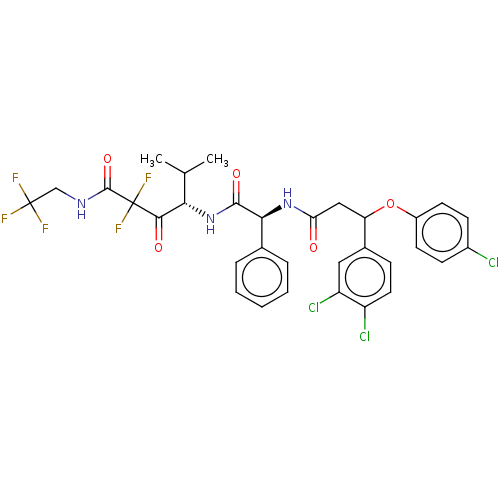 (US10428108, Example 72 | US10428108, Example 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478057 (CHEMBL256059) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477145 (CHEMBL235648) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273103 (3-[(3-methylbenzene)sulfonyl]-1-({4-[({[(3-methylb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415659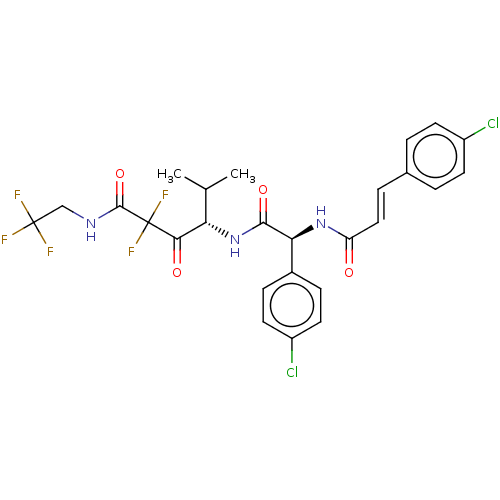 (US10428108, Example 69) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415683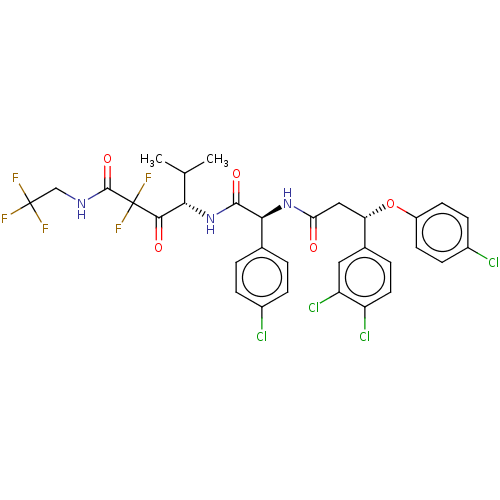 (US10428108, Example 92) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477147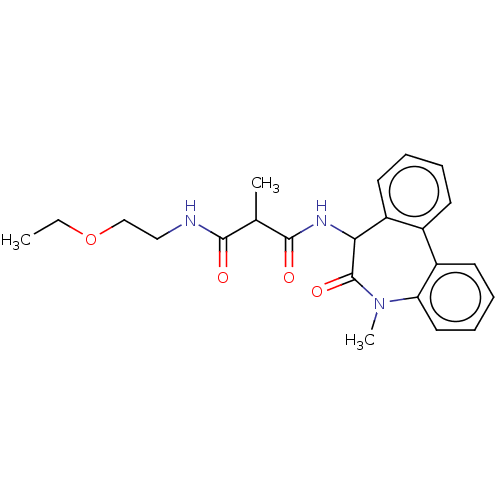 (CHEMBL235216) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477146 (CHEMBL428374) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415661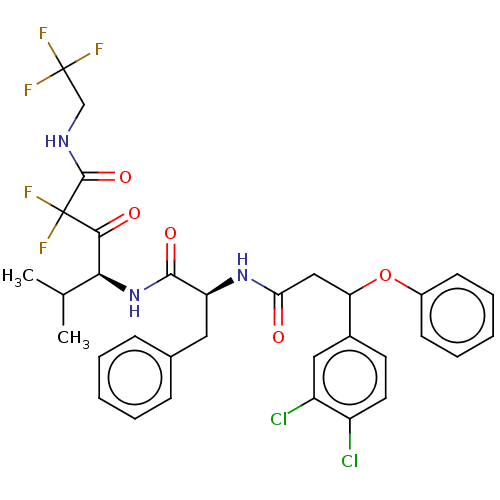 (US10428108, Example 71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477137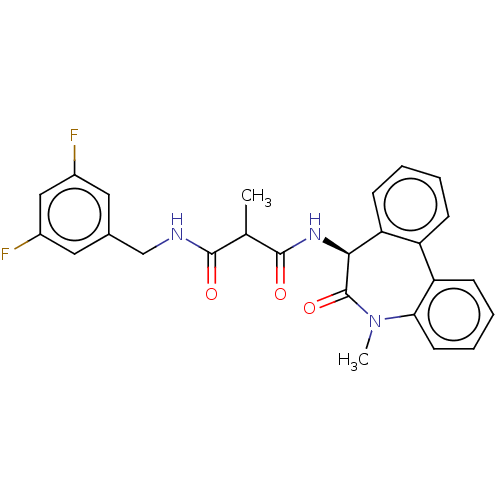 (CHEMBL235427) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477147 (CHEMBL235216) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478077 (CHEMBL403332) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415671 (US10428108, Example 81) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415680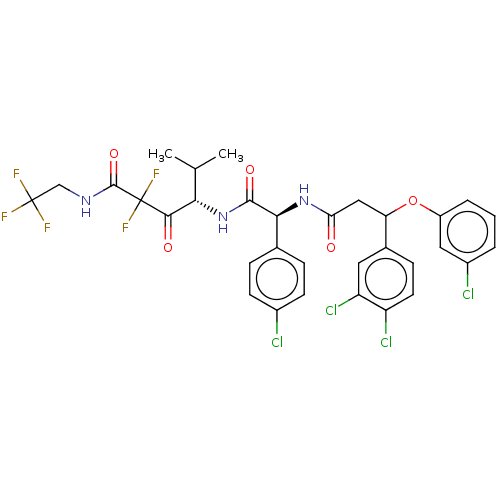 (US10428108, Example 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273102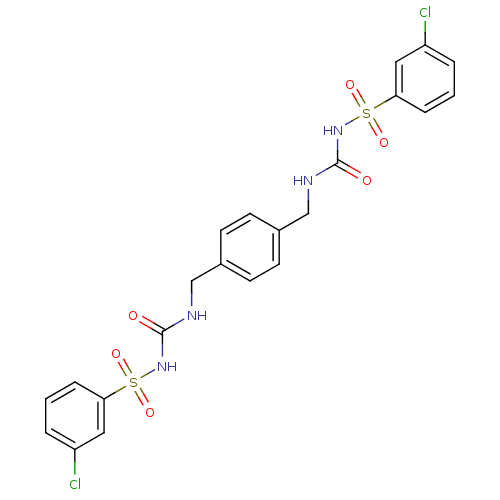 (3-[(3-chlorobenzene)sulfonyl]-1-({4-[({[(3-chlorob...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50272993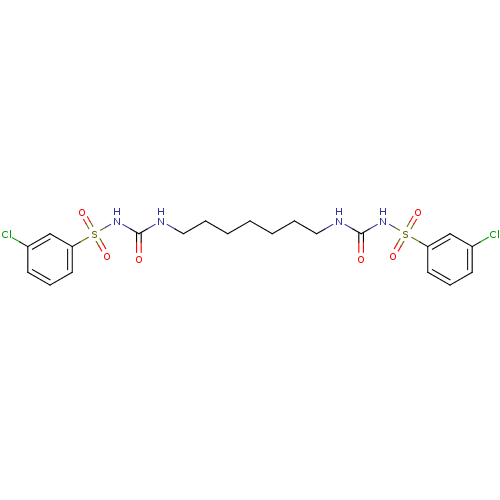 (1-[(3-chlorobenzene)sulfonyl]-3-[7-({[(3-chloroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50272994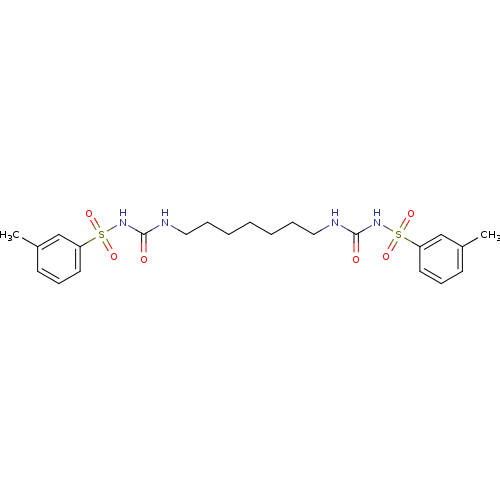 (1-[(3-methylbenzene)sulfonyl]-3-[7-({[(3-methylben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477149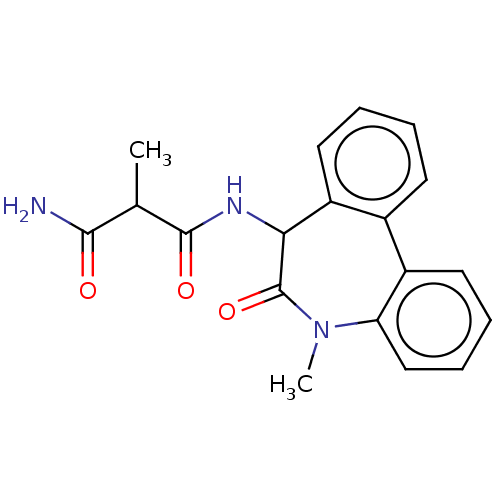 (CHEMBL430169) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477132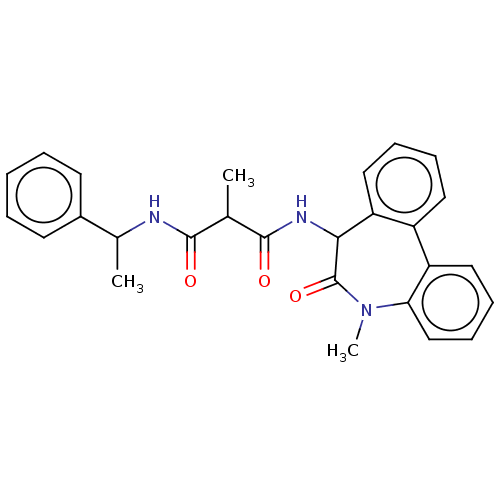 (CHEMBL267960) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478058 (CHEMBL429938) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273102 (3-[(3-chlorobenzene)sulfonyl]-1-({4-[({[(3-chlorob...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273104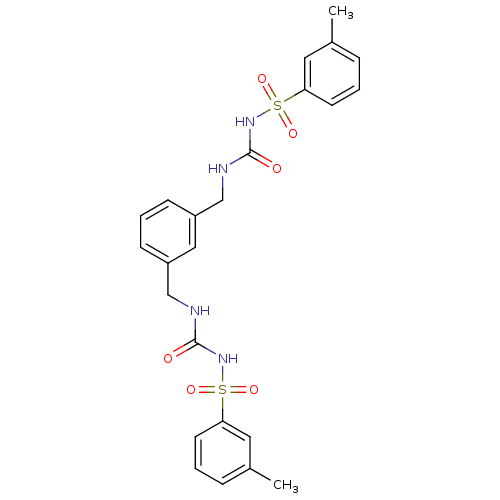 (3-[(3-methylbenzene)sulfonyl]-1-({3-[({[(3-methylb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477132 (CHEMBL267960) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50159366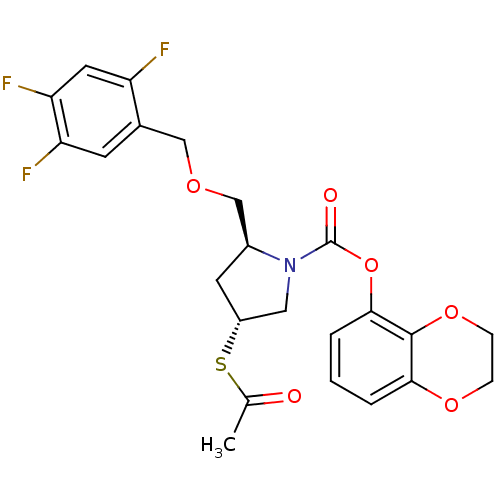 ((2S,4R)-4-Acetylsulfanyl-2-(2,4,5-trifluoro-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University Institute of Pathology Curated by ChEMBL | Assay Description Inhibitory concentration against human ECE-1 by RIA | J Med Chem 48: 483-98 (2005) Article DOI: 10.1021/jm040857x BindingDB Entry DOI: 10.7270/Q2VM4D1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415690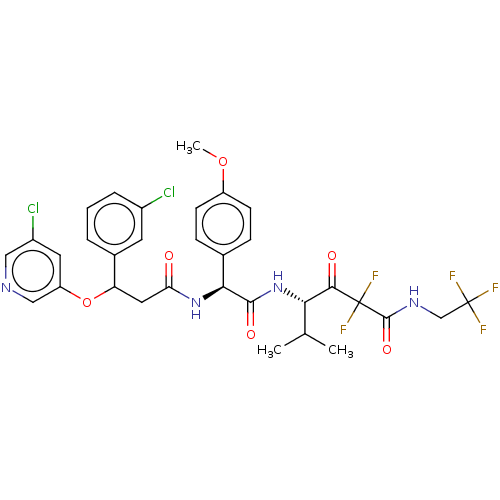 (US10428108, Example 98) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415665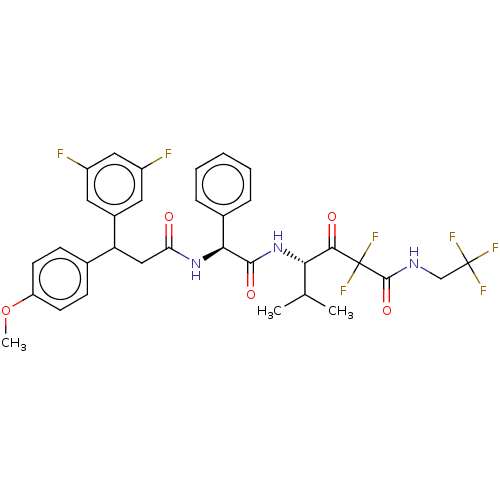 (US10428108, Example 75) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477140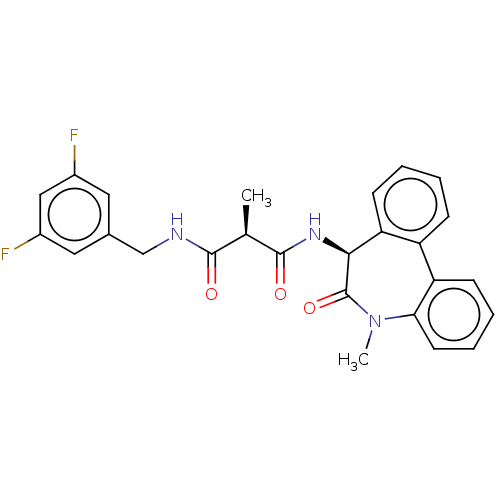 (CHEMBL267959) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415663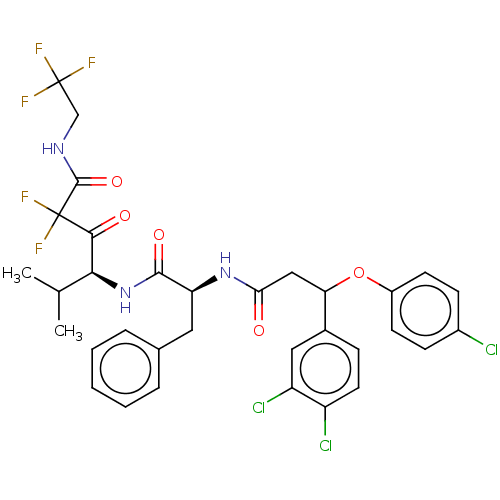 (US10428108, Example 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50272991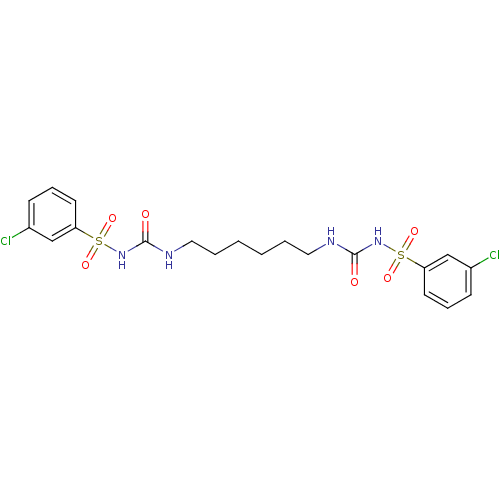 (1-[(3-chlorobenzene)sulfonyl]-3-[6-({[(3-chloroben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415667 (US10428108, Example 77 | US10428108, Example 79) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50478061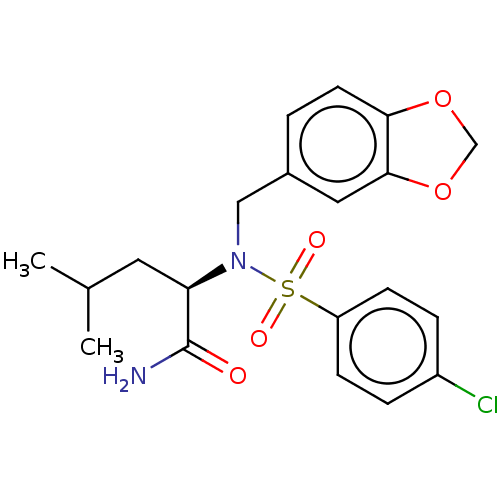 (CHEMBL439242) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells assessed as reduction of amyloid beta level by ELISA | Bioorg Med Chem Lett 18: 304-8 (2008) Article DOI: 10.1016/j.bmcl.2007.10.074 BindingDB Entry DOI: 10.7270/Q2J105X3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fructose-1,6-bisphosphatase 1 (Homo sapiens (Human)) | BDBM50273035 (3-[(3-methylbenzene)sulfonyl]-1-[8-({[(3-methylben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human liver fructose-1,6-bisphosphatase in presence of fructose-2,6-bisphosphate | Bioorg Med Chem Lett 18: 4708-12 (2008) Article DOI: 10.1016/j.bmcl.2008.06.103 BindingDB Entry DOI: 10.7270/Q26W99W7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease HTRA1 (Homo sapiens (Human)) | BDBM415655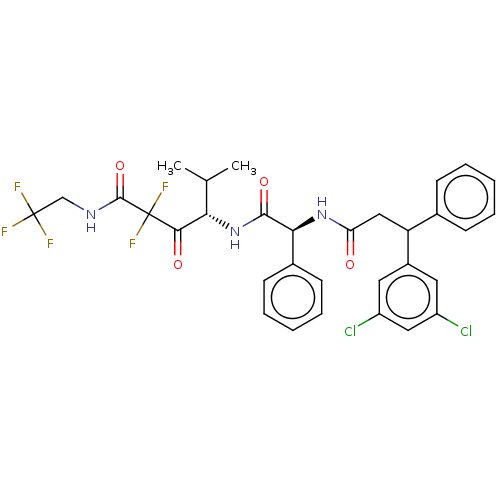 (US10428108, Example 65 | US10428108, Example 74 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w... | US Patent US10428108 (2019) BindingDB Entry DOI: 10.7270/Q2JQ13DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477142 (CHEMBL235226) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477140 (CHEMBL267959) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase assessed as amyloid-beta40 peptide production in HEK293 cells by ELISA | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477137 (CHEMBL235427) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of human gamma secretase in HEK293 cells by reporter gene assay | Bioorg Med Chem Lett 17: 5918-23 (2007) Article DOI: 10.1016/j.bmcl.2007.07.078 BindingDB Entry DOI: 10.7270/Q28S4SNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 459 total ) | Next | Last >> |