 Found 704 hits with Last Name = 'koch' and Initial = 'g'
Found 704 hits with Last Name = 'koch' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50240611

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(C)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O5/c1-11-6-8-12(9-7-11)14(13(10-23)16(24)22-27)17(25)21-15(18(26)20-5)19(2,3)4/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-2 (MMP-2) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50473686
(CHEMBL433314)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CO)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O5/c1-9(2)7-10(11(8-20)14(22)19-24)13(21)18-12(15(23)17-6)16(3,4)5/h9-12,20,24H,7-8H2,1-6H3,(H,17,23)(H,18,21)(H,19,22)/t10-,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-2 (MMP-2) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50183784
((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-2 (MMP-2) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50473686

(CHEMBL433314)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CO)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O5/c1-9(2)7-10(11(8-20)14(22)19-24)13(21)18-12(15(23)17-6)16(3,4)5/h9-12,20,24H,7-8H2,1-6H3,(H,17,23)(H,18,21)(H,19,22)/t10-,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-1 (MMP-1) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50183784

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-2 (MMP-2) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-1 (MMP-1) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50183784

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50183784

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(OC)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O6/c1-19(2,3)15(18(26)20-4)21-17(25)14(13(10-23)16(24)22-27)11-6-8-12(28-5)9-7-11/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-1 (MMP-1) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50240611

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(C)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O5/c1-11-6-8-12(9-7-11)14(13(10-23)16(24)22-27)17(25)21-15(18(26)20-5)19(2,3)4/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-1 (MMP-1) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50473685
(CHEMBL76297)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C18H27N3O5/c1-18(2,3)14(17(25)19-4)20-16(24)13(11-8-6-5-7-9-11)12(10-22)15(23)21-26/h5-9,12-14,22,26H,10H2,1-4H3,(H,19,25)(H,20,24)(H,21,23)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-1 (MMP-1) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50240611

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(C)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O5/c1-11-6-8-12(9-7-11)14(13(10-23)16(24)22-27)17(25)21-15(18(26)20-5)19(2,3)4/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50473685

(CHEMBL76297)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C18H27N3O5/c1-18(2,3)14(17(25)19-4)20-16(24)13(11-8-6-5-7-9-11)12(10-22)15(23)21-26/h5-9,12-14,22,26H,10H2,1-4H3,(H,19,25)(H,20,24)(H,21,23)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-2 (MMP-2) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50473685

(CHEMBL76297)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C18H27N3O5/c1-18(2,3)14(17(25)19-4)20-16(24)13(11-8-6-5-7-9-11)12(10-22)15(23)21-26/h5-9,12-14,22,26H,10H2,1-4H3,(H,19,25)(H,20,24)(H,21,23)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50473686

(CHEMBL433314)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CO)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O5/c1-9(2)7-10(11(8-20)14(22)19-24)13(21)18-12(15(23)17-6)16(3,4)5/h9-12,20,24H,7-8H2,1-6H3,(H,17,23)(H,18,21)(H,19,22)/t10-,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50473685

(CHEMBL76297)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccccc1)C(C)(C)C Show InChI InChI=1S/C18H27N3O5/c1-18(2,3)14(17(25)19-4)20-16(24)13(11-8-6-5-7-9-11)12(10-22)15(23)21-26/h5-9,12-14,22,26H,10H2,1-4H3,(H,19,25)(H,20,24)(H,21,23)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50473686

(CHEMBL433314)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](CO)C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H31N3O5/c1-9(2)7-10(11(8-20)14(22)19-24)13(21)18-12(15(23)17-6)16(3,4)5/h9-12,20,24H,7-8H2,1-6H3,(H,17,23)(H,18,21)(H,19,22)/t10-,11+,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50240611

((2S,3R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxo...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@@H]([C@H](CO)C(=O)NO)c1ccc(C)cc1)C(C)(C)C |r| Show InChI InChI=1S/C19H29N3O5/c1-11-6-8-12(9-7-11)14(13(10-23)16(24)22-27)17(25)21-15(18(26)20-5)19(2,3)4/h6-9,13-15,23,27H,10H2,1-5H3,(H,20,26)(H,21,25)(H,22,24)/t13-,14+,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Preclinical Research Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory potency against Matrix metalloprotease-3 (MMP-3) |
J Med Chem 45: 2289-93 (2002)
Article DOI: 10.1021/jm0110993
BindingDB Entry DOI: 10.7270/Q2HQ42NC |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50395076
(CHEMBL2164243)Show InChI InChI=1S/C6H12N2O3/c1-8(2)7-5(9)3-4-6(10)11/h3-4H2,1-2H3,(H,7,9)(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of human KDM2A expressed in Escherichia coli using 2-oxoglutarate by enzyme kinetic assay |
J Med Chem 55: 6639-43 (2012)
Article DOI: 10.1021/jm300677j
BindingDB Entry DOI: 10.7270/Q2JH3N9S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50395076

(CHEMBL2164243)Show InChI InChI=1S/C6H12N2O3/c1-8(2)7-5(9)3-4-6(10)11/h3-4H2,1-2H3,(H,7,9)(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human KDM2A expressed in Escherichia coli assessed inhibition constant for compound-enzyme-substrate complex using methyl ly... |
J Med Chem 55: 6639-43 (2012)
Article DOI: 10.1021/jm300677j
BindingDB Entry DOI: 10.7270/Q2JH3N9S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546172

(CHEMBL4762397)Show SMILES CN(Cc1c[nH]c2ncnc(-c3cccc(NC(=O)c4ccc(cc4)C(C)(C)C)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546180
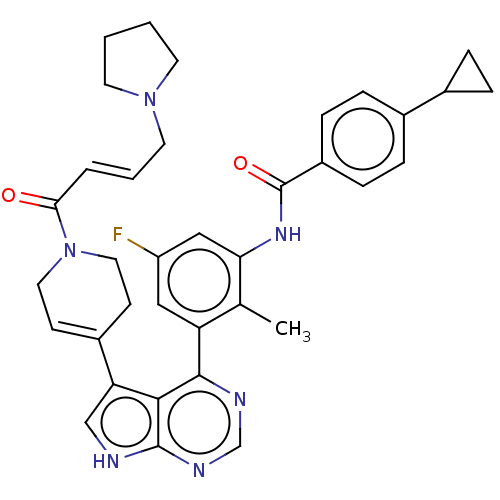
(CHEMBL4749522)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)\C=C\CN3CCCC3)c12 |t:31| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200783
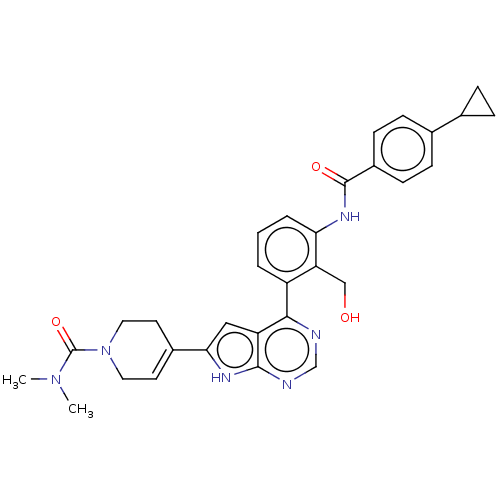
(US9233111, 17)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cccc(NC(=O)c2ccc(cc2)C2CC2)c1CO |c:8| Show InChI InChI=1S/C31H32N6O3/c1-36(2)31(40)37-14-12-21(13-15-37)27-16-24-28(32-18-33-29(24)34-27)23-4-3-5-26(25(23)17-38)35-30(39)22-10-8-20(9-11-22)19-6-7-19/h3-5,8-12,16,18-19,38H,6-7,13-15,17H2,1-2H3,(H,35,39)(H,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BMX (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200756
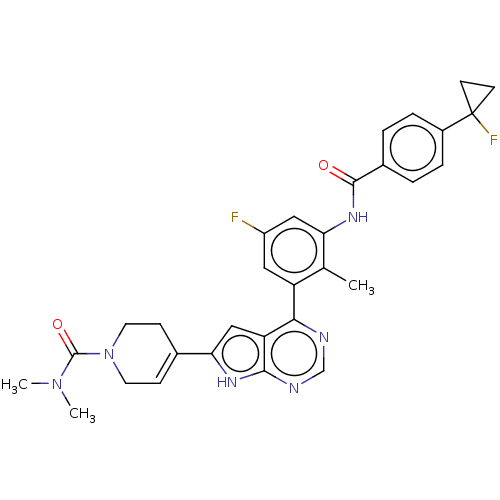
(US9233111, 1)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc(cc2)C2(F)CC2)c1C |c:8| Show InChI InChI=1S/C31H30F2N6O2/c1-18-23(14-22(32)15-25(18)37-29(40)20-4-6-21(7-5-20)31(33)10-11-31)27-24-16-26(36-28(24)35-17-34-27)19-8-12-39(13-9-19)30(41)38(2)3/h4-8,14-17H,9-13H2,1-3H3,(H,37,40)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348537

(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546179

(CHEMBL4739958)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3CCN(CC3)C(=O)C=C)c12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546175

(CHEMBL4795673)Show SMILES CN(C(=O)Cc1c[nH]c2ncnc(-c3cc(F)cc(NC(=O)c4ccc(cc4)C4CC4)c3C)c12)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50546176

(CHEMBL4746262)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(c12)C1(O)CN(C1)C(=O)C=C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00317
BindingDB Entry DOI: 10.7270/Q20K2D40 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200888
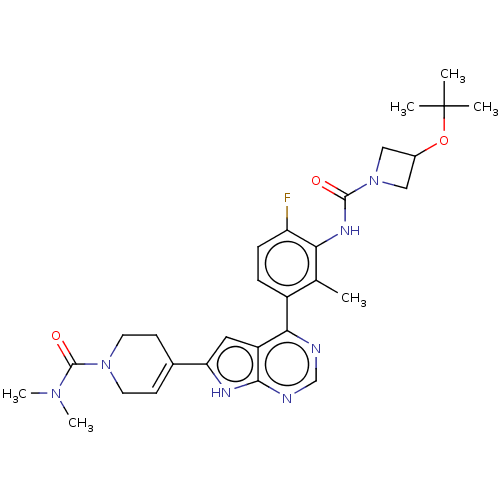
(US9233111, 63)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1ccc(F)c(NC(=O)N2CC(C2)OC(C)(C)C)c1C |c:8| Show InChI InChI=1S/C29H36FN7O3/c1-17-20(7-8-22(30)24(17)34-27(38)37-14-19(15-37)40-29(2,3)4)25-21-13-23(33-26(21)32-16-31-25)18-9-11-36(12-10-18)28(39)35(5)6/h7-9,13,16,19H,10-12,14-15H2,1-6H3,(H,34,38)(H,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348534
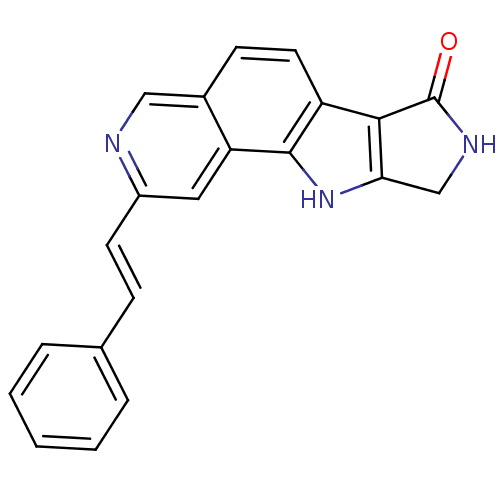
(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200778

(US9233111, 16)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cccc(NC(=O)c2ccc(cc2F)C(C)(C)O)c1CO |c:8| Show InChI InChI=1S/C31H33FN6O4/c1-31(2,42)19-8-9-21(24(32)14-19)29(40)36-25-7-5-6-20(23(25)16-39)27-22-15-26(35-28(22)34-17-33-27)18-10-12-38(13-11-18)30(41)37(3)4/h5-10,14-15,17,39,42H,11-13,16H2,1-4H3,(H,36,40)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200860

(US9233111, 47)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cccc(N2CCc3cc(ccc3C2=O)C(C)(C)O)c1CO |c:8| Show InChI InChI=1S/C33H36N6O4/c1-33(2,43)22-8-9-23-21(16-22)12-15-39(31(23)41)28-7-5-6-24(26(28)18-40)29-25-17-27(36-30(25)35-19-34-29)20-10-13-38(14-11-20)32(42)37(3)4/h5-10,16-17,19,40,43H,11-15,18H2,1-4H3,(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200887

(US9233111, 62)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1ccc(F)c(NC(=O)N2CC(C2)OC(C)(C)C)c1 |c:8| Show InChI InChI=1S/C28H34FN7O3/c1-28(2,3)39-19-14-36(15-19)26(37)33-23-12-18(6-7-21(23)29)24-20-13-22(32-25(20)31-16-30-24)17-8-10-35(11-9-17)27(38)34(4)5/h6-8,12-13,16,19H,9-11,14-15H2,1-5H3,(H,33,37)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200766

(US9233111, 4)Show SMILES CC(C)N(C)c1ccc(cc1)C(=O)Nc1cc(F)cc(c1C)-c1ncnc2[nH]c(cc12)C1=CCN(CC1)C(=O)N(C)C |t:35| Show InChI InChI=1S/C32H36FN7O2/c1-19(2)39(6)24-9-7-22(8-10-24)31(41)37-27-16-23(33)15-25(20(27)3)29-26-17-28(36-30(26)35-18-34-29)21-11-13-40(14-12-21)32(42)38(4)5/h7-11,15-19H,12-14H2,1-6H3,(H,37,41)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200881
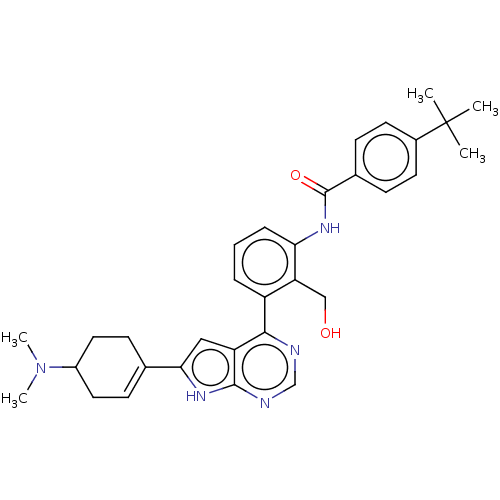
(US9233111, 56)Show SMILES CN(C)C1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cccc(NC(=O)c2ccc(cc2)C(C)(C)C)c1CO |c:6| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200802

(US9233111, 25)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C(C)(C)C)cc(F)cc1-c1ncnc2[nH]c(cc12)C1=CCS(=O)CC1 |t:34| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200768

(US9233111, 6)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc(cc2)C(C)(C)CO)c1C |c:8| Show InChI InChI=1S/C32H35FN6O3/c1-19-24(14-23(33)15-26(19)37-30(41)21-6-8-22(9-7-21)32(2,3)17-40)28-25-16-27(36-29(25)35-18-34-28)20-10-12-39(13-11-20)31(42)38(4)5/h6-10,14-16,18,40H,11-13,17H2,1-5H3,(H,37,41)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200770
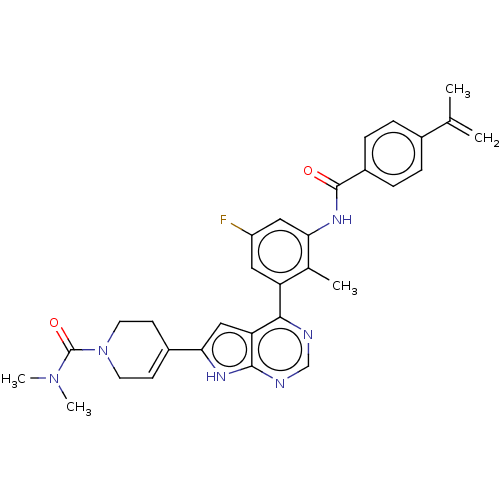
(US9233111, 8)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc(cc2)C(C)=C)c1C |c:8| Show InChI InChI=1S/C31H31FN6O2/c1-18(2)20-6-8-22(9-7-20)30(39)36-26-15-23(32)14-24(19(26)3)28-25-16-27(35-29(25)34-17-33-28)21-10-12-38(13-11-21)31(40)37(4)5/h6-10,14-17H,1,11-13H2,2-5H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200769
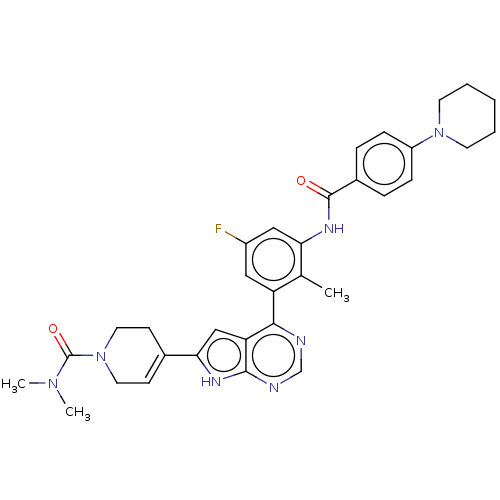
(US9233111, 7)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc(cc2)N2CCCCC2)c1C |c:8| Show InChI InChI=1S/C33H36FN7O2/c1-21-26(17-24(34)18-28(21)38-32(42)23-7-9-25(10-8-23)40-13-5-4-6-14-40)30-27-19-29(37-31(27)36-20-35-30)22-11-15-41(16-12-22)33(43)39(2)3/h7-11,17-20H,4-6,12-16H2,1-3H3,(H,38,42)(H,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200767

(US9233111, 5)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc3c(C)c[nH]c3c2)c1C |c:8| Show InChI InChI=1S/C31H30FN7O2/c1-17-15-33-27-11-20(5-6-22(17)27)30(40)37-25-13-21(32)12-23(18(25)2)28-24-14-26(36-29(24)35-16-34-28)19-7-9-39(10-8-19)31(41)38(3)4/h5-7,11-16,33H,8-10H2,1-4H3,(H,37,40)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200884

(US9233111, 59)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)N2Cc3ccc(F)cc3C2)c1C |c:8| Show InChI InChI=1S/C30H29F2N7O2/c1-17-23(11-22(32)12-25(17)36-29(40)39-14-19-4-5-21(31)10-20(19)15-39)27-24-13-26(35-28(24)34-16-33-27)18-6-8-38(9-7-18)30(41)37(2)3/h4-6,10-13,16H,7-9,14-15H2,1-3H3,(H,36,40)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
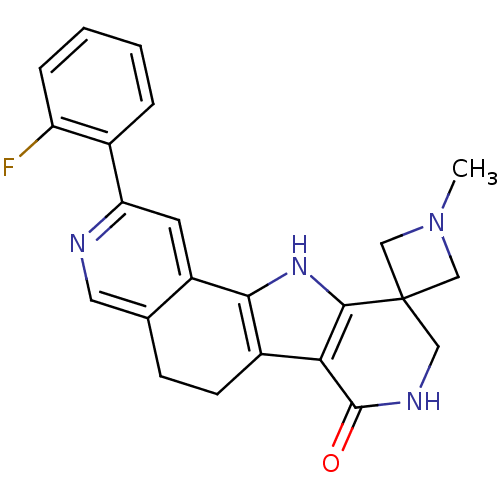
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200817

(US9233111, 31)Show SMILES CC(=O)OCC(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C |c:10| Show InChI InChI=1S/C32H30FN5O4/c1-18-25(13-24(33)14-27(18)37-32(41)23-7-5-21(6-8-23)20-3-4-20)30-26-15-28(36-31(26)35-17-34-30)22-9-11-38(12-10-22)29(40)16-42-19(2)39/h5-9,13-15,17,20H,3-4,10-12,16H2,1-2H3,(H,37,41)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50075752

(4-[5-(4-Chloro-phenyl)-3-(2-isobutoxy-phenyl)-1H-p...)Show SMILES CC(C)COc1ccccc1-c1cc([nH]c1-c1ccncc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H23ClN2O/c1-17(2)16-29-24-6-4-3-5-21(24)22-15-23(18-7-9-20(26)10-8-18)28-25(22)19-11-13-27-14-12-19/h3-15,17,28H,16H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity determined by reduction in binding of 125 I-glucagon to the human glucagon receptor expressed on CHO cells in absence of Mg+2 |
Bioorg Med Chem Lett 9: 641-6 (1999)
BindingDB Entry DOI: 10.7270/Q2VD6XM1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM200764

(US9233111, 2)Show SMILES CN(C)C(=O)N1CCC(=CC1)c1cc2c(ncnc2[nH]1)-c1cc(F)cc(NC(=O)c2ccc3c(OCC3(C)C)c2)c1C |c:8| Show InChI InChI=1S/C32H33FN6O3/c1-18-22(13-21(33)14-25(18)37-30(40)20-6-7-24-27(12-20)42-16-32(24,2)3)28-23-15-26(36-29(23)35-17-34-28)19-8-10-39(11-9-19)31(41)38(4)5/h6-8,12-15,17H,9-11,16H2,1-5H3,(H,37,40)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG
US Patent
| Assay Description
The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... |
US Patent US9233111 (2016)
BindingDB Entry DOI: 10.7270/Q2ZS2V9C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data