

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101813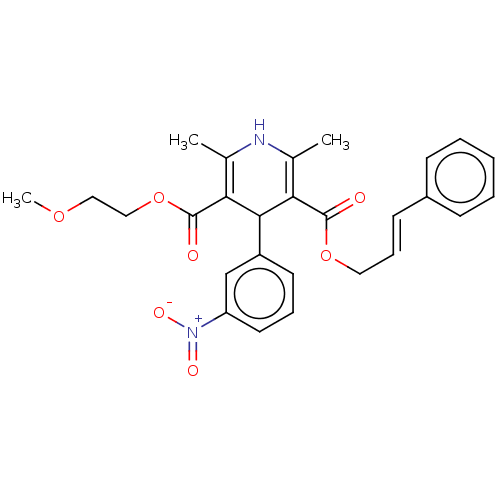 (CHEBI:31399 | Cilnidipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibition of voltage-dependent L-type calcium channel in rat thoracic aorta ring assessed as effect on high K+ induced contraction by Magnus method | Bioorg Med Chem Lett 18: 4813-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.096 BindingDB Entry DOI: 10.7270/Q208684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101813 (CHEBI:31399 | Cilnidipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Pharmaceuticals Co., Ltd Curated by ChEMBL | Assay Description Inhibition of Voltage-dependent L-type calcium channel in rat thoracic aorta ring | Bioorg Med Chem Lett 21: 3317-9 (2011) Article DOI: 10.1016/j.bmcl.2011.04.007 BindingDB Entry DOI: 10.7270/Q2N019CJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101813 (CHEBI:31399 | Cilnidipine) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421355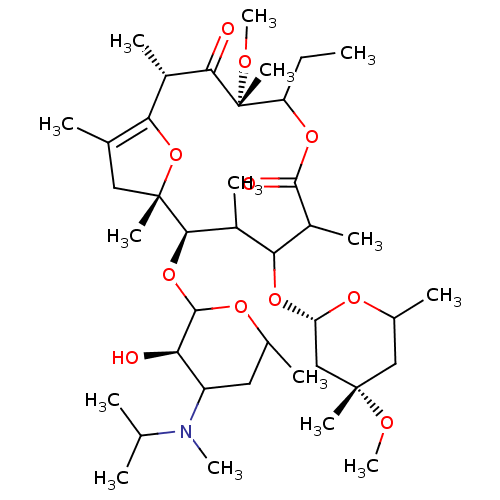 (CHEMBL289955) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421358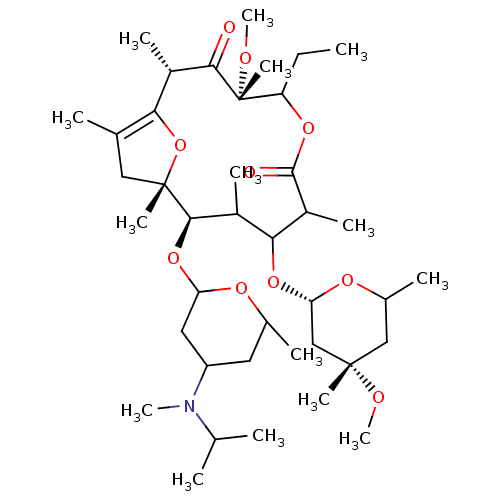 (CHEMBL45141) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421357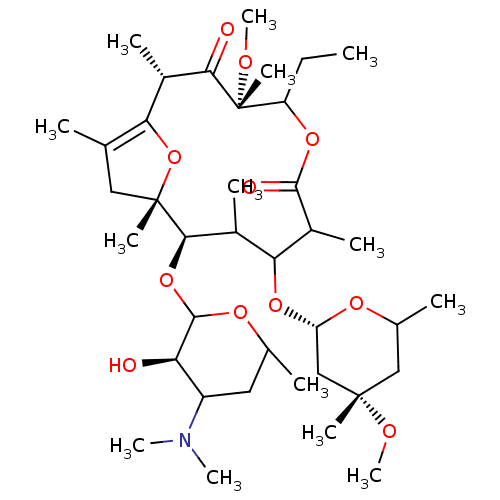 (CHEMBL297168) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421355 (CHEMBL289955) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475660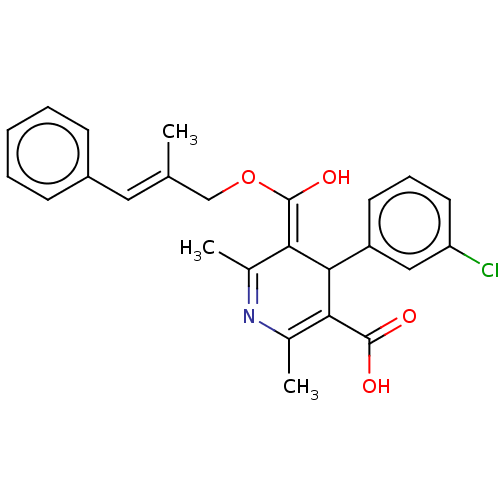 (CHEMBL201566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421354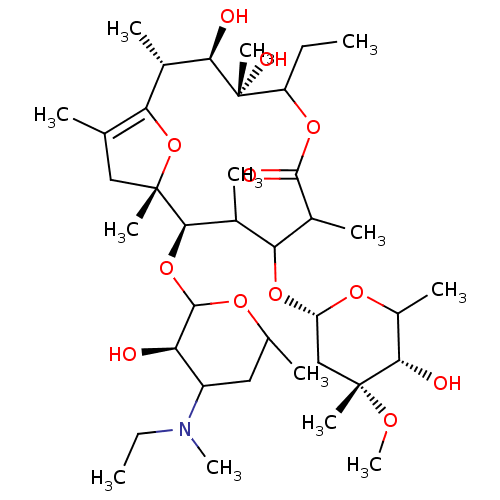 (CHEMBL26966) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro motilin receptor binding, expressed as negative logarithm of IC50 | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421354 (CHEMBL26966) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421356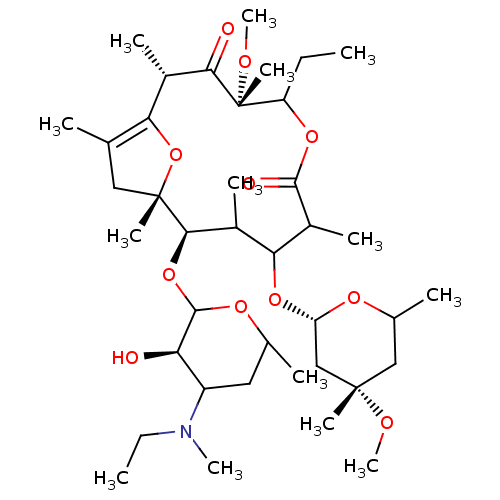 (CHEMBL43313) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475658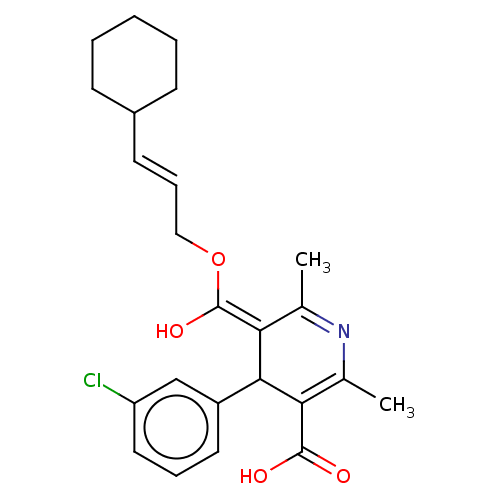 (CHEMBL201984) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421349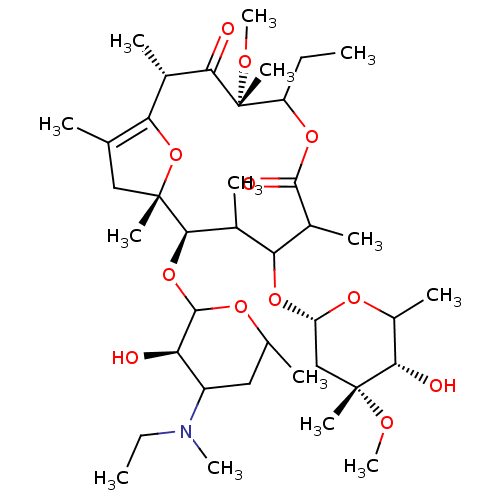 (CHEMBL265495) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421349 (CHEMBL265495) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro motilin receptor binding, expressed as negative logarithm of IC50 | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421356 (CHEMBL43313) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421357 (CHEMBL297168) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5052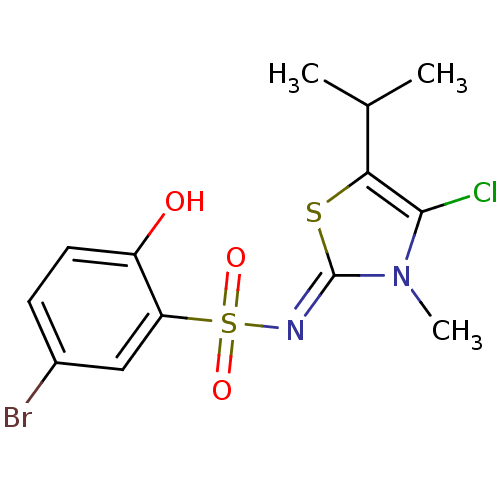 (5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475653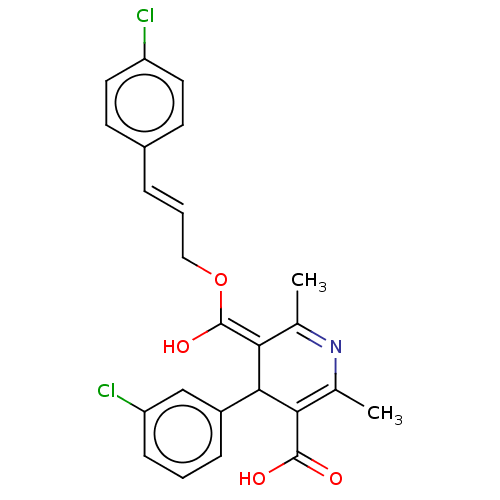 (CHEMBL382528) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421351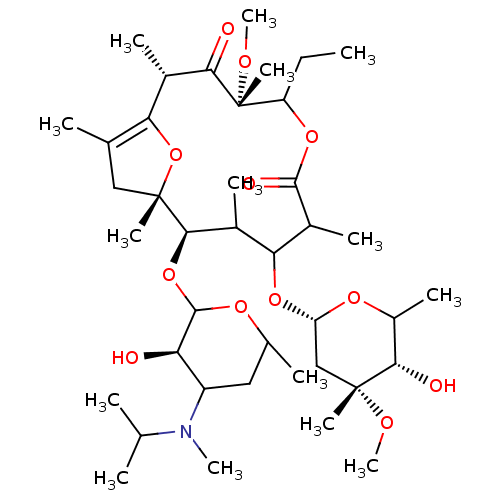 (CHEMBL27424) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro motilin receptor binding, expressed as negative logarithm of IC50 | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421351 (CHEMBL27424) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421349 (CHEMBL265495) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 2.5 | 25 |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro acid stability with hydrochloric acid solution (pH 2.5 ) at room temperature for 2 hr by assaying the solution fo... | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421349 (CHEMBL265495) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475650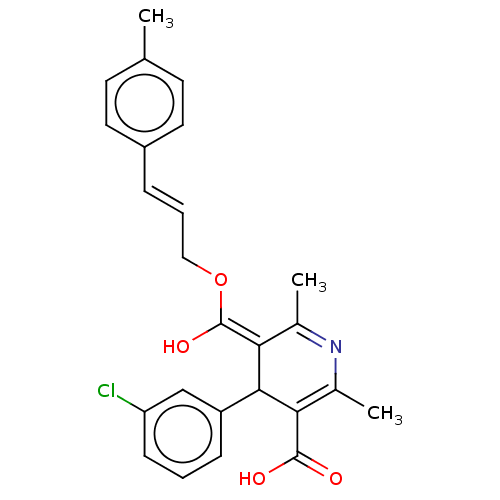 (CHEMBL203041) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 12: 6171-82 (2004) Article DOI: 10.1016/j.bmc.2004.08.050 BindingDB Entry DOI: 10.7270/Q23R0R24 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479255 (CHEMBL453084) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibition of voltage-dependent L-type calcium channel in rat thoracic aorta ring assessed as effect on high K+ induced contraction by Magnus method | Bioorg Med Chem Lett 18: 4813-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.096 BindingDB Entry DOI: 10.7270/Q208684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421351 (CHEMBL27424) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 2.5 | 25 |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro acid stability with hydrochloric acid solution (pH 2.5 ) at room temperature for 2 hr by assaying the solution fo... | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421351 (CHEMBL27424) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421350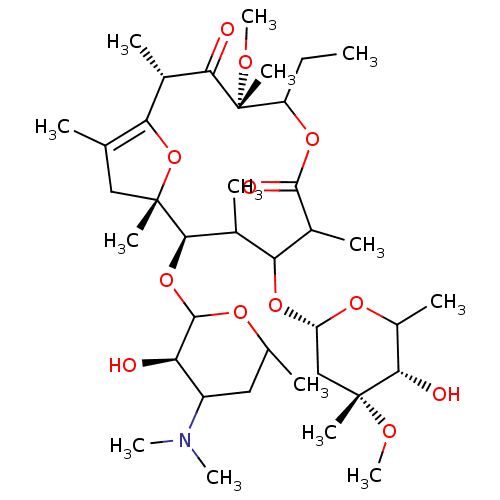 (CHEMBL27419) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined after treatment with hydrochloric acid in rabbit small intestinal smooth muscle tis... | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421350 (CHEMBL27419) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 2.5 | 25 |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro acid stability with hydrochloric acid solution (pH 2.5 ) at room temperature for 2 hr by assaying the solution fo... | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421350 (CHEMBL27419) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 2.5 | 25 |
TBA Curated by ChEMBL | Assay Description Concentration required for in vitro acid stability with hydrochloric acid solution (pH 2.5 ) at room temperature for 2 hr by assaying the solution fo... | Bioorg Med Chem Lett 4: 1347-1352 (1994) Article DOI: 10.1016/S0960-894X(01)80359-0 BindingDB Entry DOI: 10.7270/Q2PR7X8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5054 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-1,3-thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475663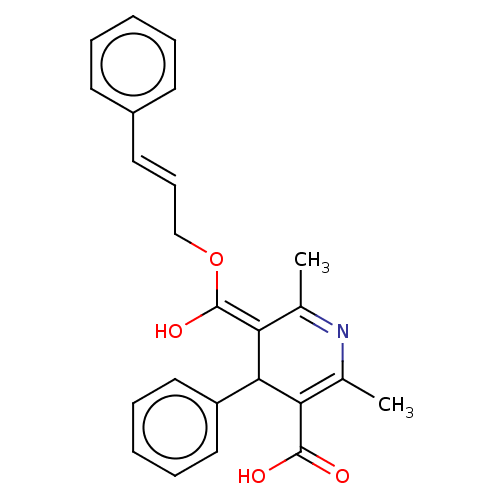 (CHEMBL202464) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Motilin receptor (Oryctolagus cuniculus) | BDBM50421350 (CHEMBL27419) | Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards motilin receptor was determined in rabbit small intestinal smooth muscle tissue homogenate | Bioorg Med Chem Lett 4: 1649-1654 (1994) Article DOI: 10.1016/S0960-894X(01)80583-7 BindingDB Entry DOI: 10.7270/Q2K075JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5051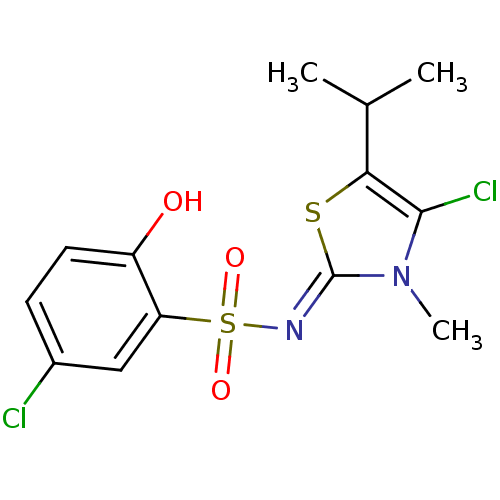 (5-Chloro-N-(4-chloro-5-isopropyl-3-methyl-13-thiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5055 (N-(4-Chloro-5-isopropyl-3-methyl-1,3-thiazol-2(3H)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475664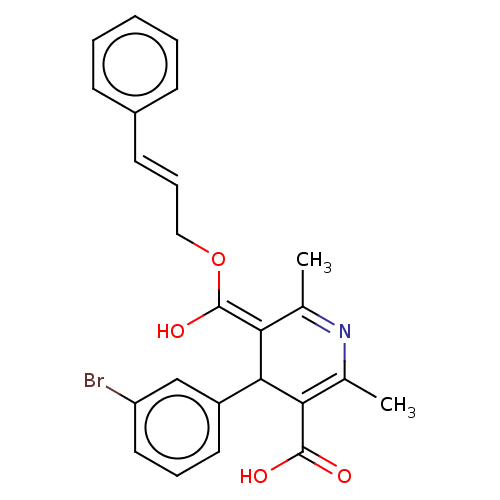 (CHEMBL369934) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5048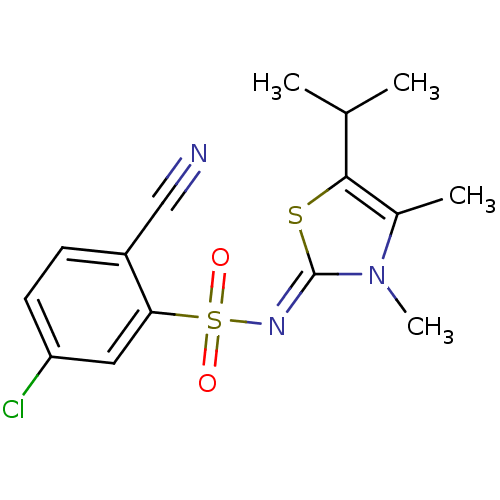 (5-Chloro-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-t...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5041 (N-(5-tert-Butyl-3,4-dimethyl-1,3-thiazol-2(3H)-yli...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5053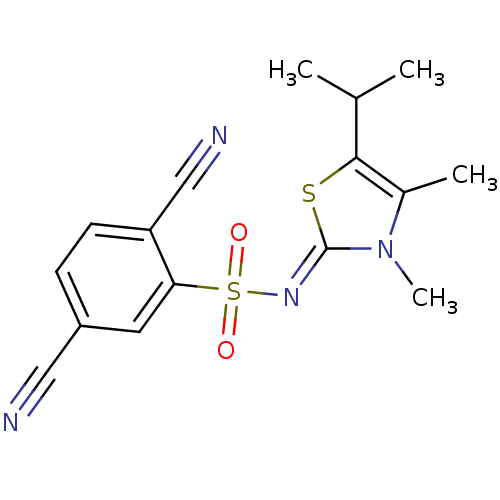 (2,5-dicyano-N-[(2Z)-3,4-dimethyl-5-(propan-2-yl)-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475672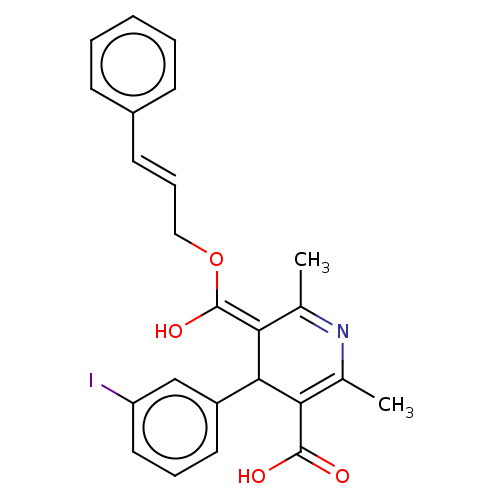 (CHEMBL201057) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM5052 (5-bromo-N-[(2Z)-4-chloro-3-methyl-5-(propan-2-yl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475662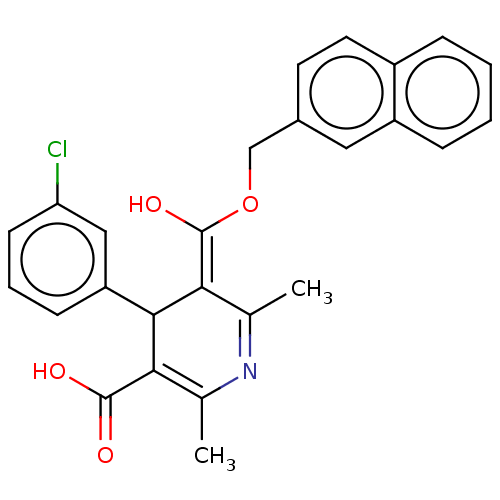 (CHEMBL201860) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM5050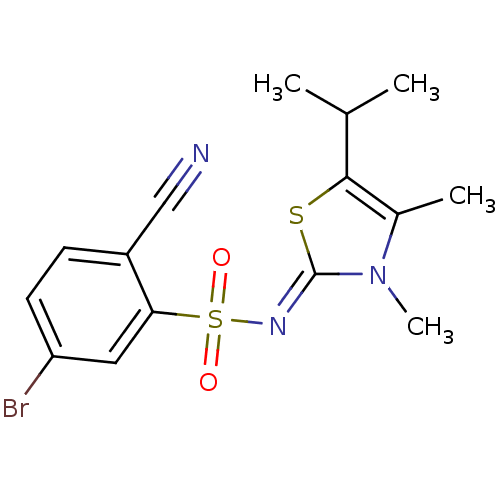 (5-Bromo-2-cyano-N-(5-isopropyl-3,4-dimethyl-1,3-th...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co. Ltd | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | Bioorg Med Chem 13: 949-61 (2005) Article DOI: 10.1016/j.bmc.2004.11.045 BindingDB Entry DOI: 10.7270/Q2C53J1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475669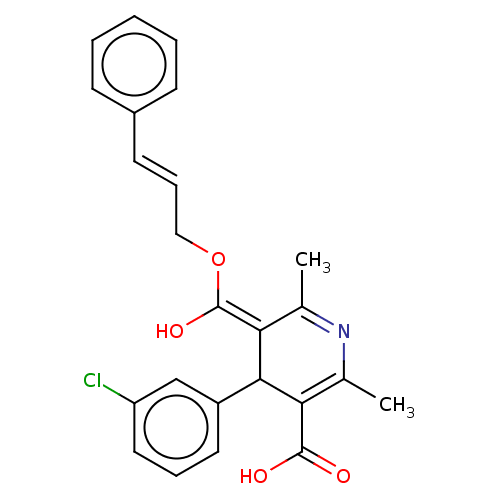 (CHEMBL382072) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibition of voltage-dependent L-type calcium channel in rat thoracic aorta ring assessed as effect on high K+ induced contraction by Magnus method | Bioorg Med Chem Lett 18: 4813-6 (2008) Article DOI: 10.1016/j.bmcl.2008.07.096 BindingDB Entry DOI: 10.7270/Q208684R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475669 (CHEMBL382072) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50475656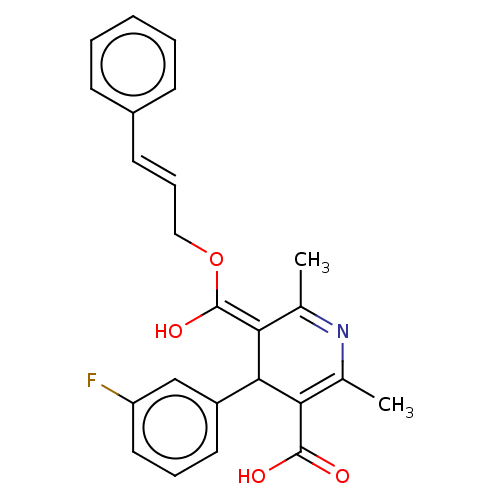 (CHEMBL201306) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-type calcium channel in SD rat thoracic aorta by Magnus method | Bioorg Med Chem Lett 16: 798-802 (2006) Article DOI: 10.1016/j.bmcl.2005.11.021 BindingDB Entry DOI: 10.7270/Q2NP276R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 266 total ) | Next | Last >> |