

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.574 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P1 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Rattus norvegicus) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.772 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P4 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 2 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P2 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | >5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P3 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116666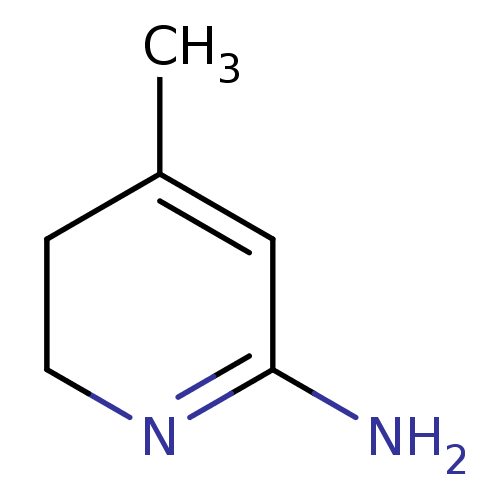 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116668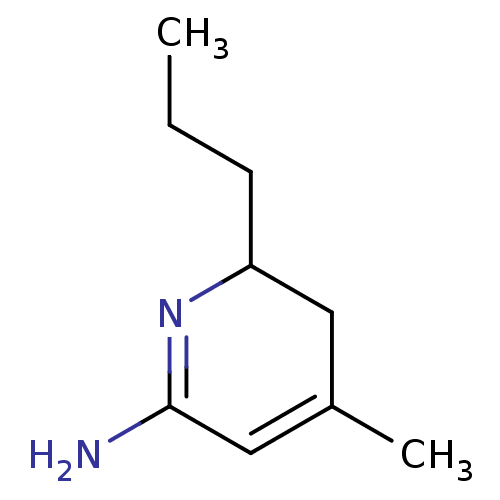 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116667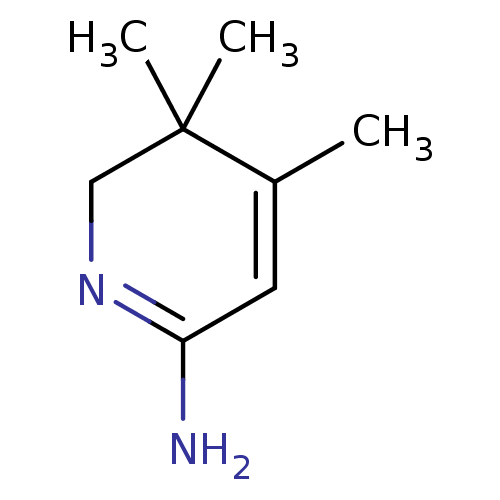 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description The concentration required for inhibition of Inducible nitric oxide synthase in mouse | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116670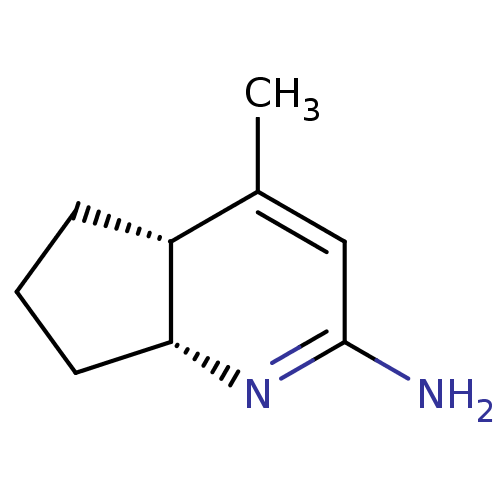 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116676 (4,6-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116673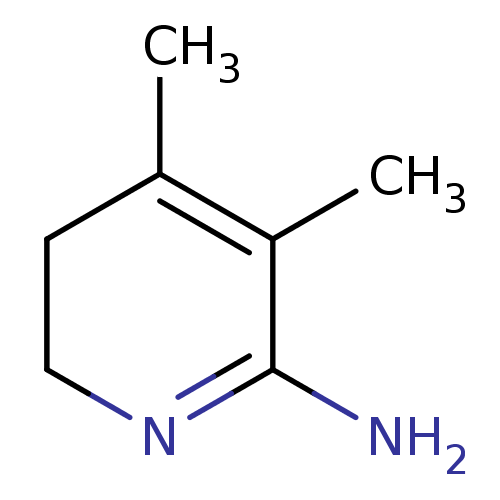 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116673 (3,4-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116674 (6-Allyl-4-methyl-5,6-dihydro-1H-pyridin-(2Z)-ylide...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116675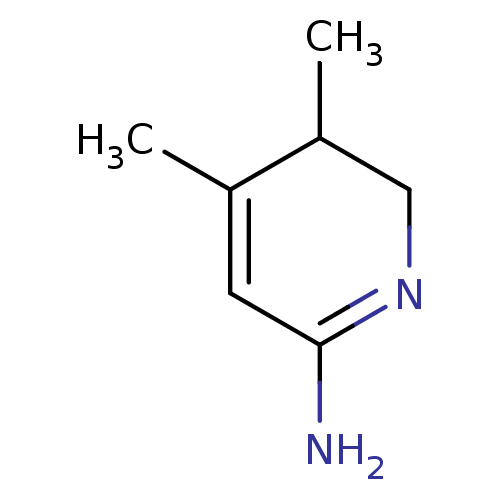 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116670 ((4aR,7aR)-4-Methyl-1,4a,5,6,7,7a-hexahydro-[1]pyri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116675 (4,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116668 (4-Methyl-6-propyl-5,6-dihydro-1H-pyridin-(2Z)-ylid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116666 (4-Methyl-3,6-dihydro-1H-pyridin-(2Z)-ylideneamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50237936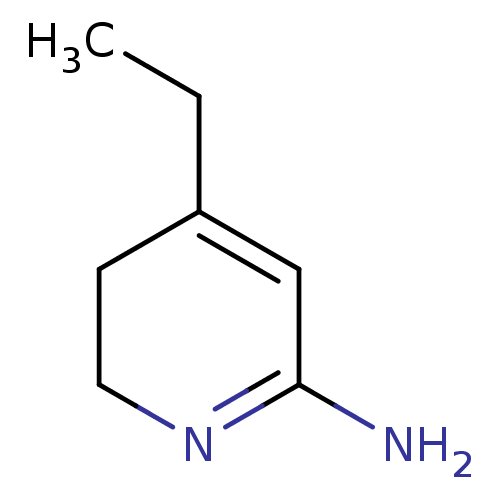 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116677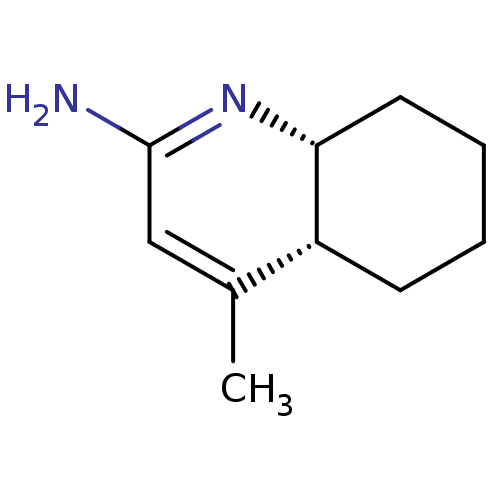 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50237936 (4-Ethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneamine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116667 (4,5,5-Trimethyl-5,6-dihydro-1H-pyridin-(2Z)-yliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50116669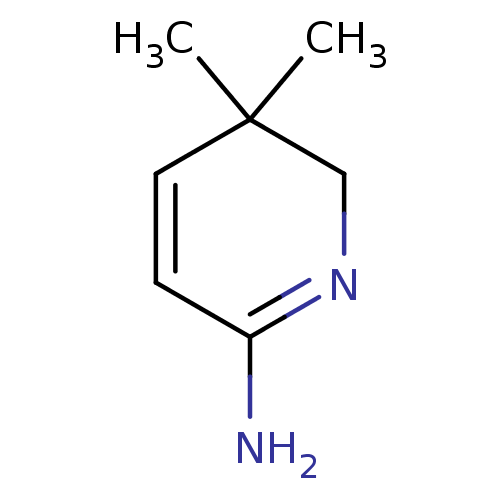 (5,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit human Inducible nitric oxide synthase over expressed in A549 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116677 ((4aR,8aR)-4-Methyl-4a,5,6,7,8,8a-hexahydro-1H-quin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM50116669 (5,5-Dimethyl-5,6-dihydro-1H-pyridin-(2Z)-ylideneam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Fukui Research Institute Curated by ChEMBL | Assay Description Inhibition of human endothelial Nitric Oxide Synthase expressed in Sf-21 cells | Bioorg Med Chem Lett 12: 2291-4 (2002) BindingDB Entry DOI: 10.7270/Q2M32V3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250632 (CHEMBL4100785) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250636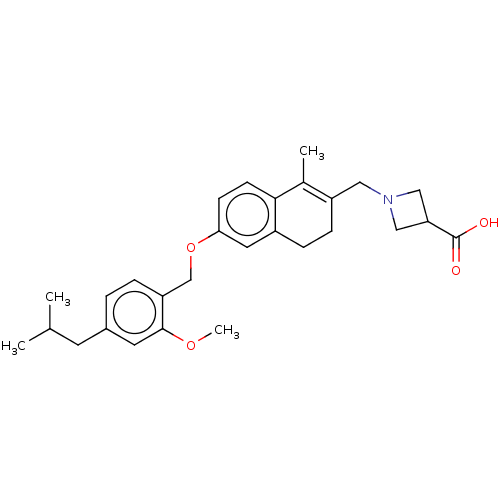 (CHEMBL4085321) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250623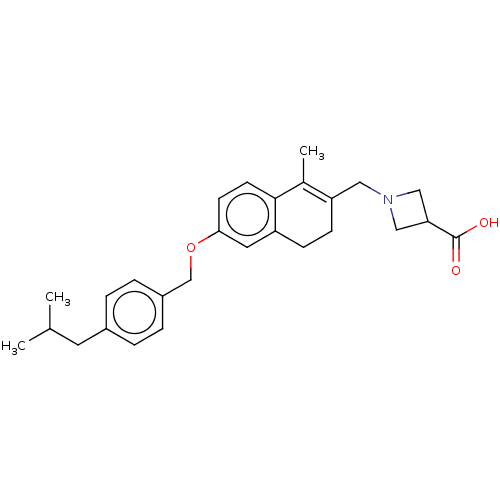 (CHEMBL4103252) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250629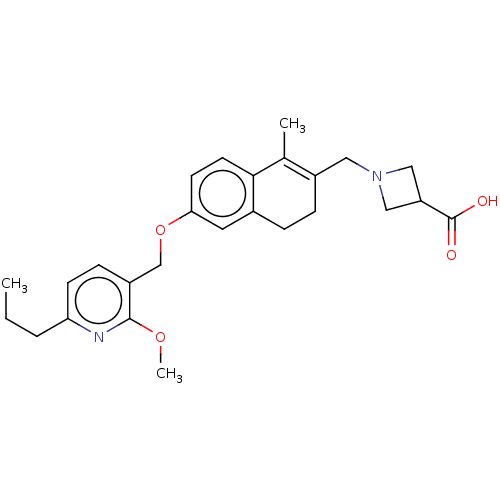 (CHEMBL4074505) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250637 (CHEMBL4087932) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250615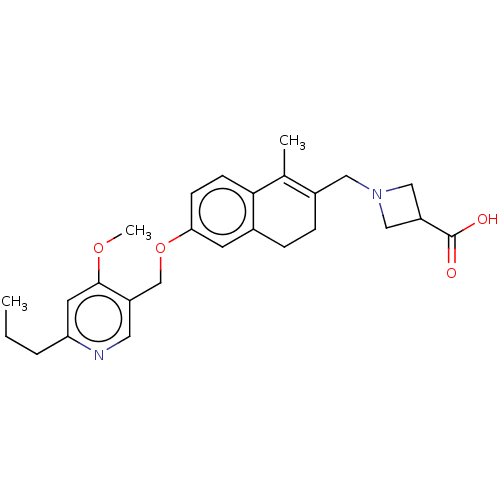 (CHEMBL4077888) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 57 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250636 (CHEMBL4085321) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Rattus norvegicus) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0286 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from rat S1P1 receptor after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250632 (CHEMBL4100785) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250637 (CHEMBL4087932) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50250614 (CHEMBL4095920) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250628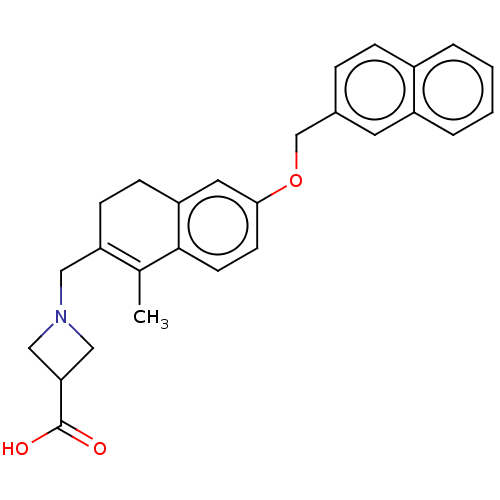 (CHEMBL4062652) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250637 (CHEMBL4087932) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P3 receptor expressed in CHO-K1 cells assessed as increase in S1P-stimulated calcium influx preincubated for 5 mins follo... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50250631 (CHEMBL4093489) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonist activity at human S1P1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by E... | J Med Chem 60: 9508-9530 (2017) Article DOI: 10.1021/acs.jmedchem.7b00785 BindingDB Entry DOI: 10.7270/Q2J968SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 86 total ) | Next | Last >> |