

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138348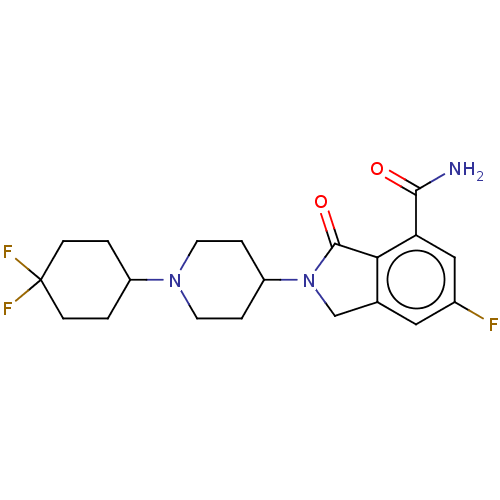 (US8877944, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167422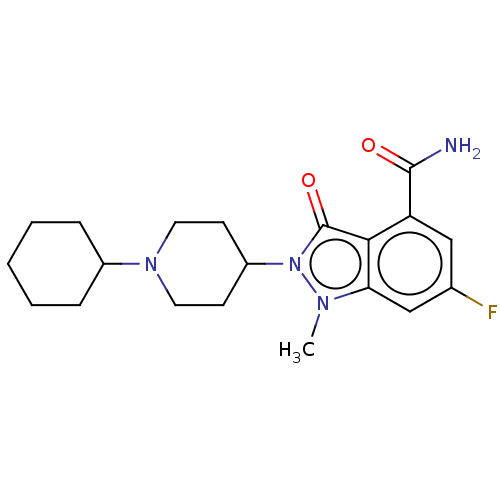 (US9073893, 22) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167419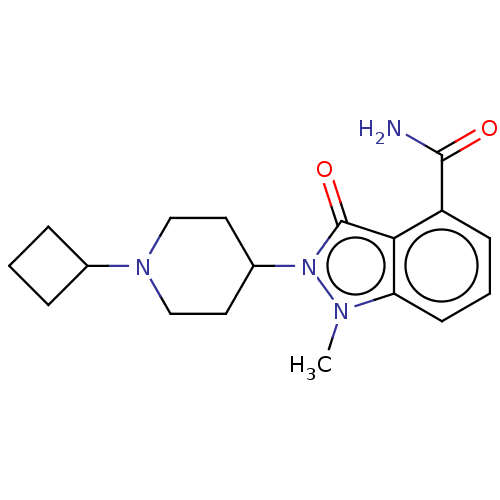 (US9073893, 18) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167412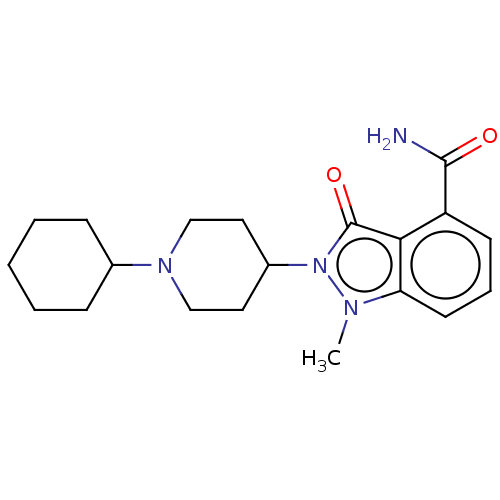 (US9073893, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138348 (US8877944, 99) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138344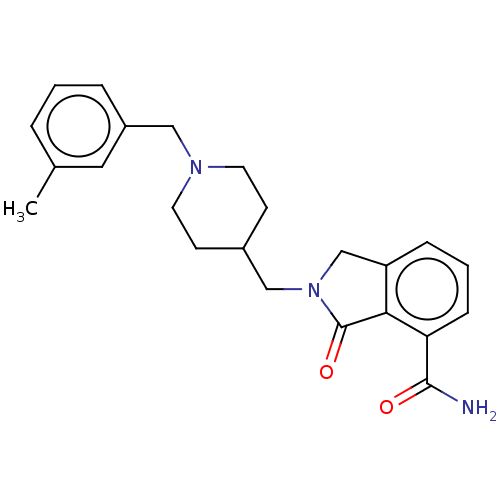 (US8877944, 49) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138344 (US8877944, 49) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167427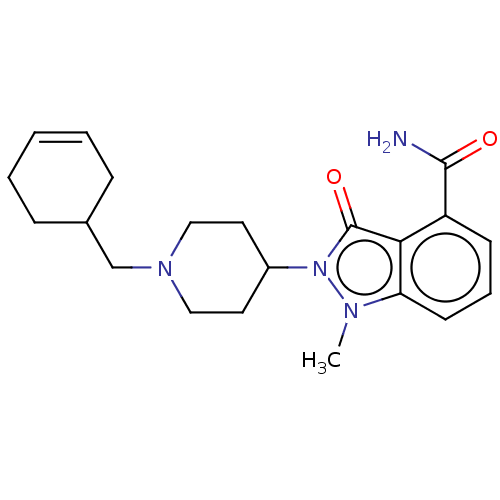 (US9073893, 65) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167416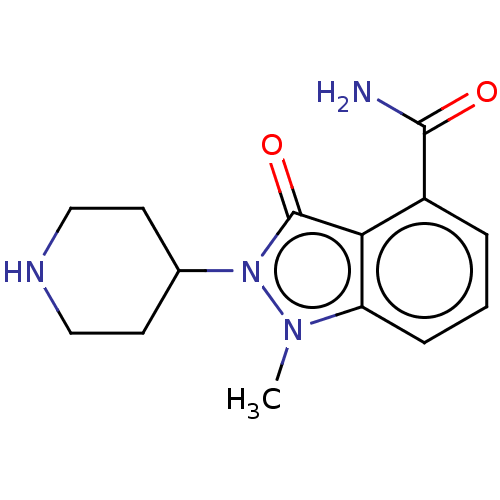 (US9073893, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167426 (US9073893, 62) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138354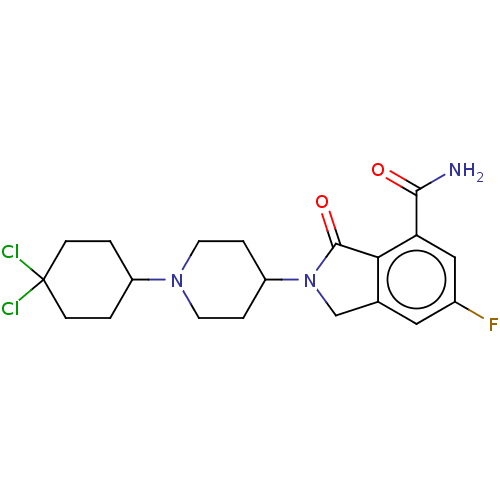 (US8877944, 105) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138342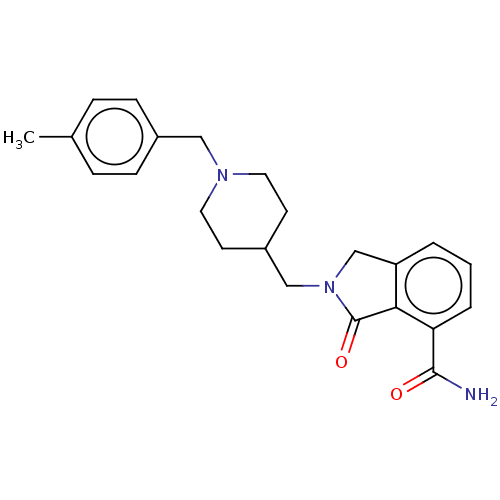 (US8877944, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138349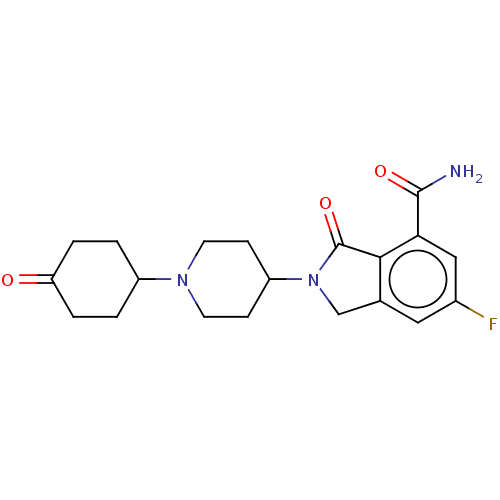 (US8877944, 103) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138354 (US8877944, 105) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138302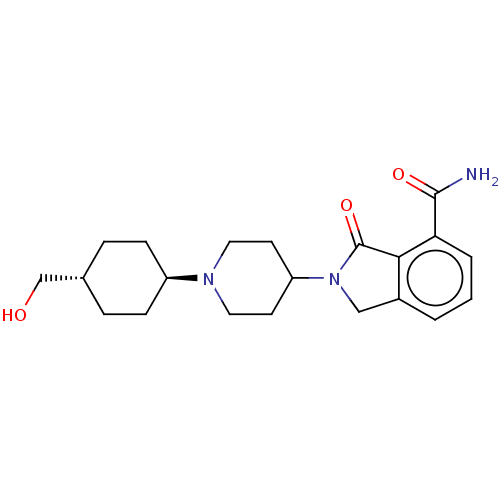 (US8877944, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167424 (US9073893, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167413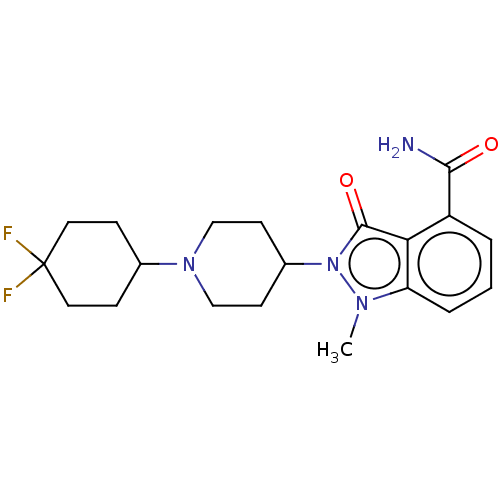 (US9073893, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138325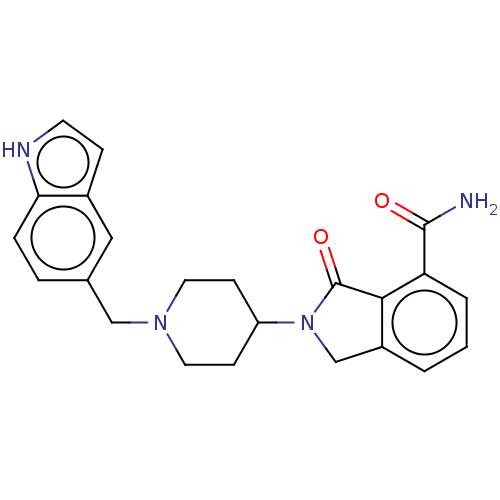 (US8877944, 29) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138325 (US8877944, 29) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138327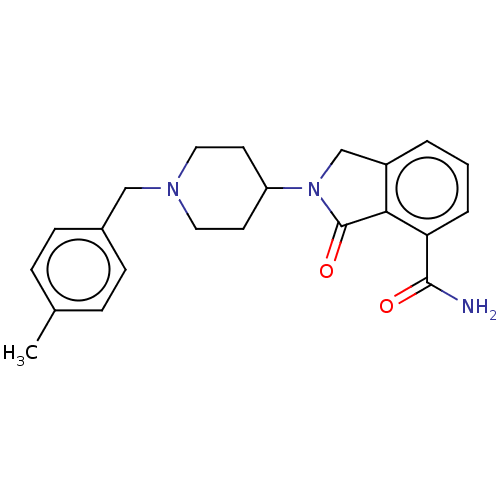 (US8877944, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138327 (US8877944, 31) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167414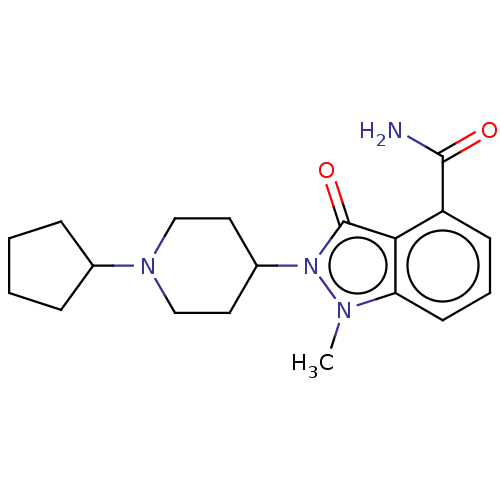 (US9073893, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167417 (US9073893, 14) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167430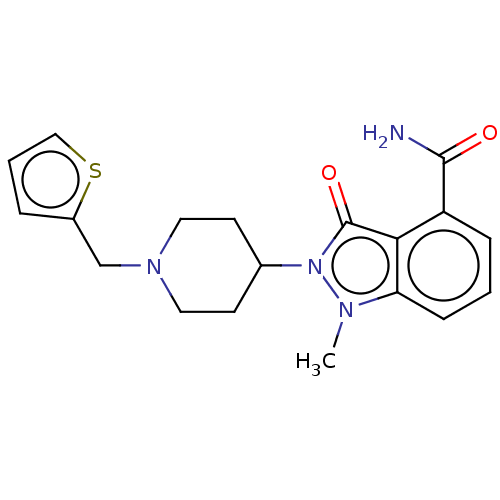 (US9073893, 63) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138301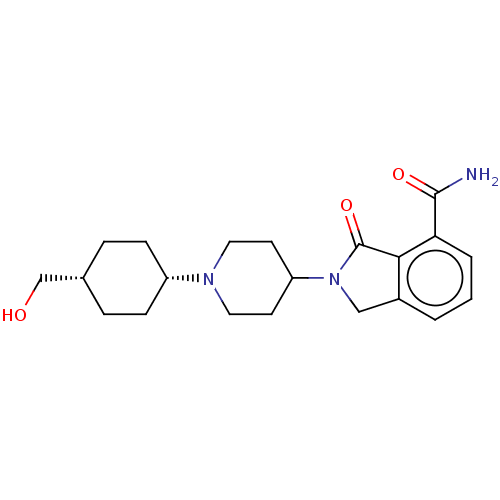 (US8877944, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167411 (US9073893, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167428 (US9073893, 64) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM138298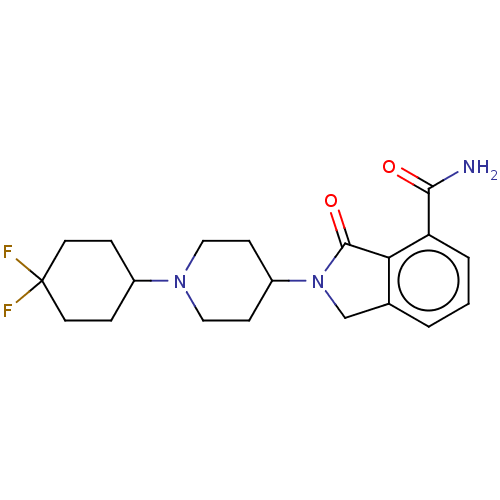 (US8877944, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138346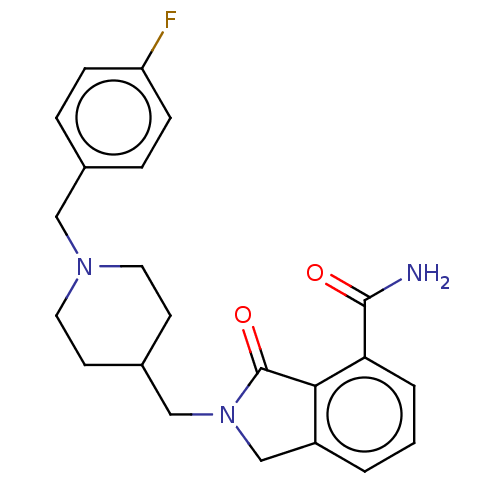 (US8877944, 51) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138300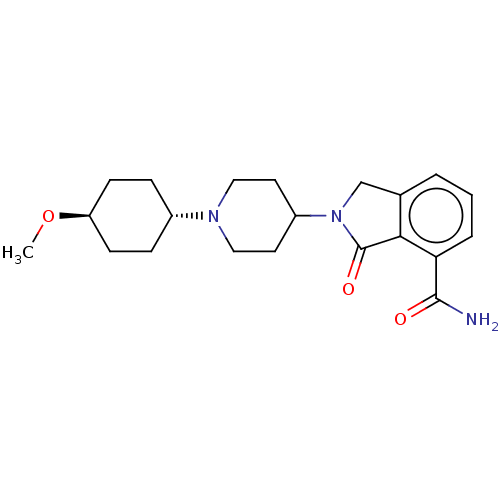 (US8877944, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138324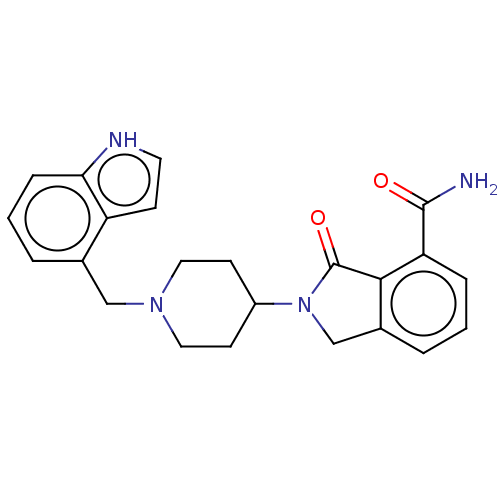 (US8877944, 28) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167425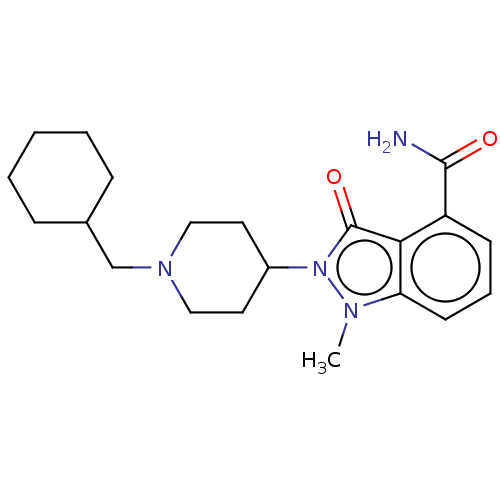 (US9073893, 33) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138322 (US8877944, 26) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50110830 (CHEMBL3605978) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167410 (US9073893, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138332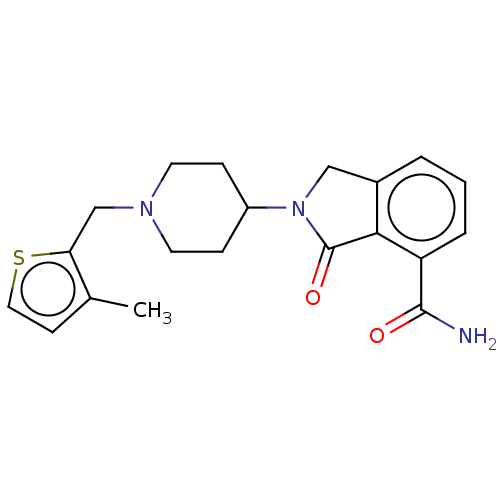 (US8877944, 36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167429 (US9073893, 66) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167409 (US9073893, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138333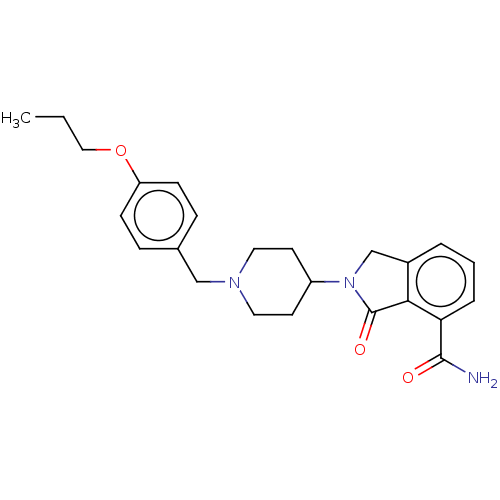 (US8877944, 37) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138334 (US8877944, 38) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138326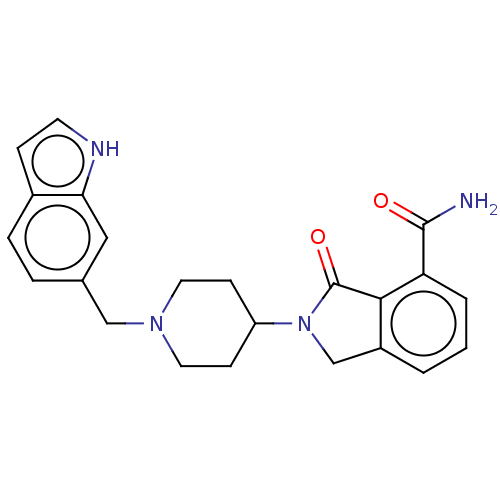 (US8877944, 30) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138331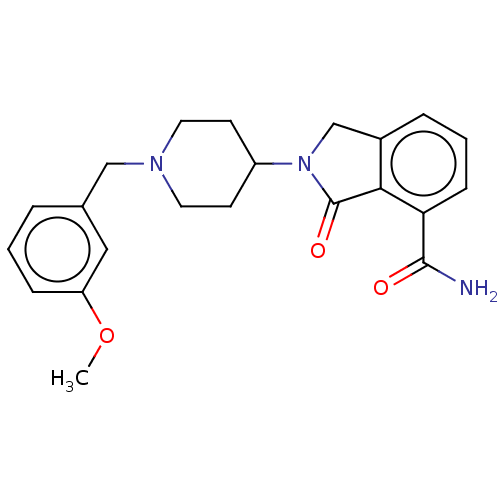 (US8877944, 35) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138323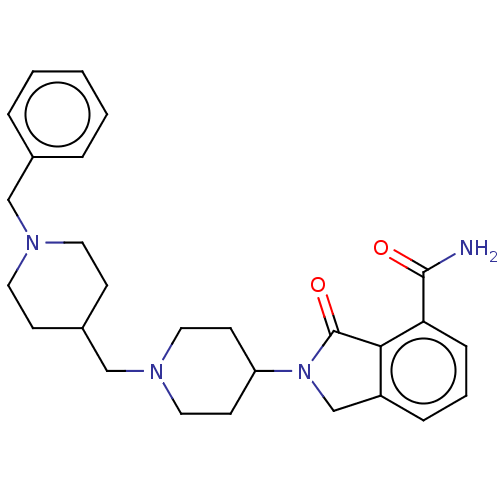 (US8877944, 27) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138335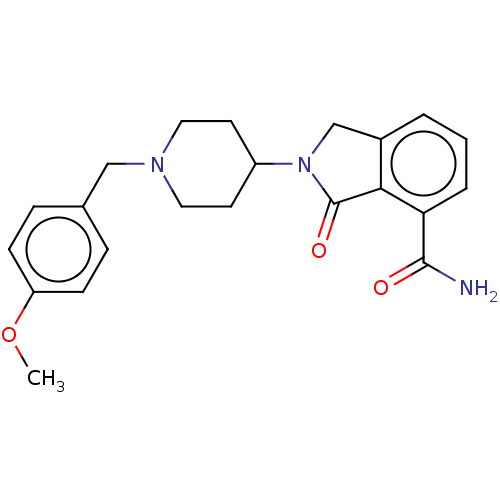 (US8877944, 39) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138298 (US8877944, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM167423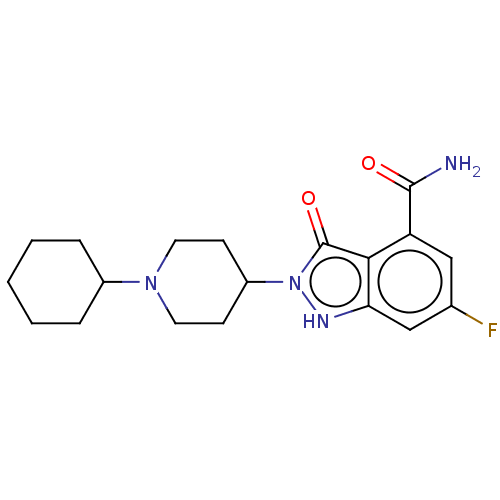 (US9073893, 23) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | 37 |
NERVIANO MEDICAL SCIENCES S.R.L. US Patent | Assay Description Cellular activity of PARP-1 inhibitors was assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC).... | US Patent US9073893 (2015) BindingDB Entry DOI: 10.7270/Q2WM1C52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138341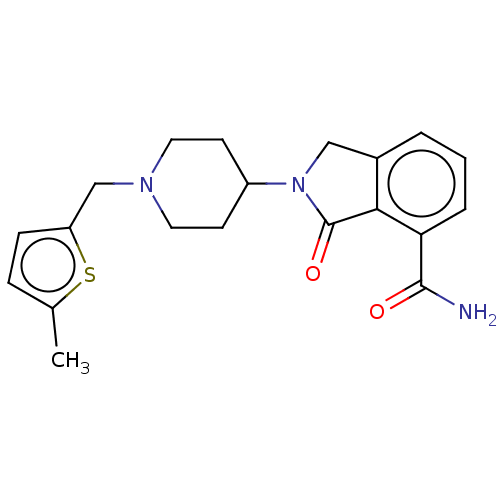 (US8877944, 45) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50110809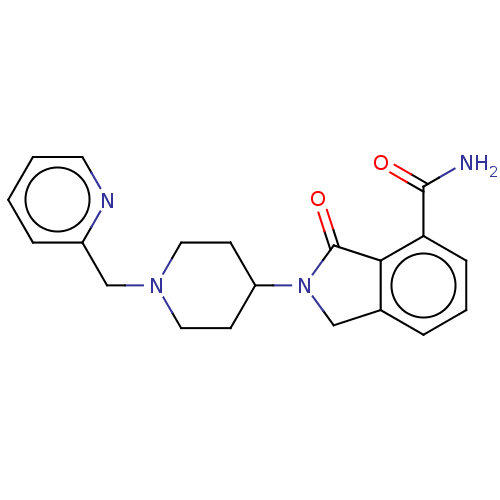 (CHEMBL3606008 | US11420940, cpd (15)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of PARP1 in human HeLa cells assessed as reduction of H2O2-induced PAR formation preincubated for 30 mins followed by H2O2 addition measur... | J Med Chem 58: 6875-98 (2015) Article DOI: 10.1021/acs.jmedchem.5b00680 BindingDB Entry DOI: 10.7270/Q2JD4ZKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138328 (US8877944, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Mus musculus) | BDBM138299 (US8877944, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences S.R.L. US Patent | Assay Description Affinity evaluation of the tested compounds and their selectivity with respect to the different PARP isoforms of interest was assessed in a displacem... | US Patent US8877944 (2014) BindingDB Entry DOI: 10.7270/Q2NG4PBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 441 total ) | Next | Last >> |