

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50403047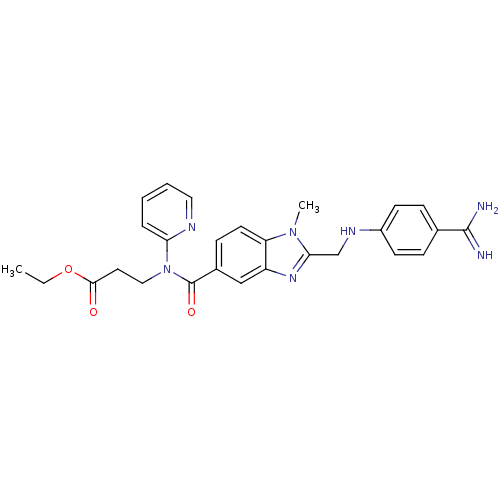 (CHEMBL1231568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO2-mediated mitomycin C metabolism using NADH as cosubstrate incubated for 5 mins prior to NADH addition measured a... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant NQO2-mediated mitomycin C metabolism using NADH as cosubstrate incubated for 5 mins prior to NADH addition measured a... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human thrombin-mediated platelet aggregation in cell-based assay | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50180953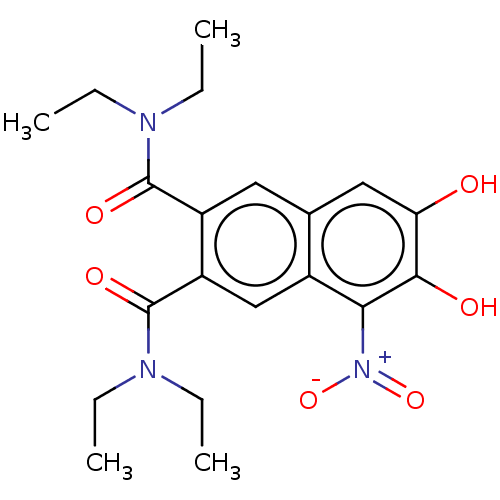 (CHEMBL3818719) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Caprotec Bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged soluble COMT expressed in Escherichia coli BL21 using aesculetin as substrate after 60 mins by microplate ... | J Med Chem 59: 4664-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01970 BindingDB Entry DOI: 10.7270/Q2WD42HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50108877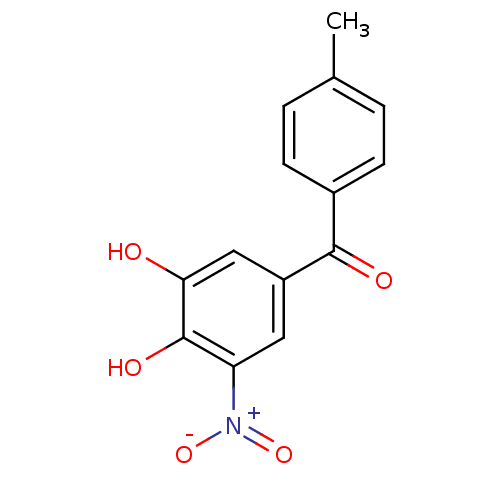 ((3,4-Dihydroxy-5-nitro-phenyl)-p-tolyl-methanone |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Caprotec Bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged soluble COMT expressed in Escherichia coli BL21 using aesculetin as substrate after 60 mins by microplate ... | J Med Chem 59: 4664-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01970 BindingDB Entry DOI: 10.7270/Q2WD42HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50180952 (CHEMBL3818350) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 179 | n/a | n/a | n/a | n/a | n/a | n/a |
Caprotec Bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged soluble COMT expressed in Escherichia coli BL21 using aesculetin as substrate after 60 mins by microplate ... | J Med Chem 59: 4664-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01970 BindingDB Entry DOI: 10.7270/Q2WD42HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50108879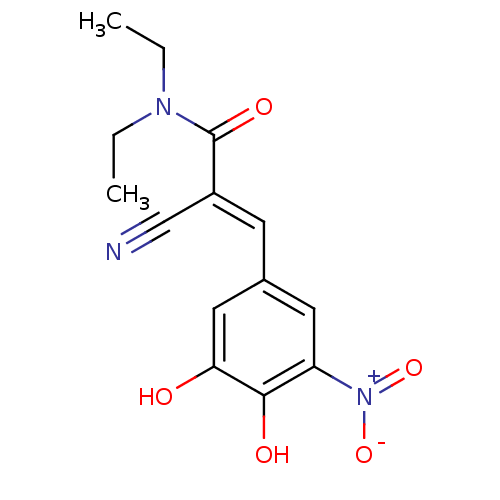 ((2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-d...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 386 | n/a | n/a | n/a | n/a | n/a | n/a |
Caprotec Bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged soluble COMT expressed in Escherichia coli BL21 using aesculetin as substrate after 60 mins by microplate ... | J Med Chem 59: 4664-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01970 BindingDB Entry DOI: 10.7270/Q2WD42HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant NQO2-mediated mitomycin C metabolism using NADH as cosubstrate incubated for 5 mins prior to NADH additio... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lanosterol synthase (Rattus norvegicus) | BDBM50180954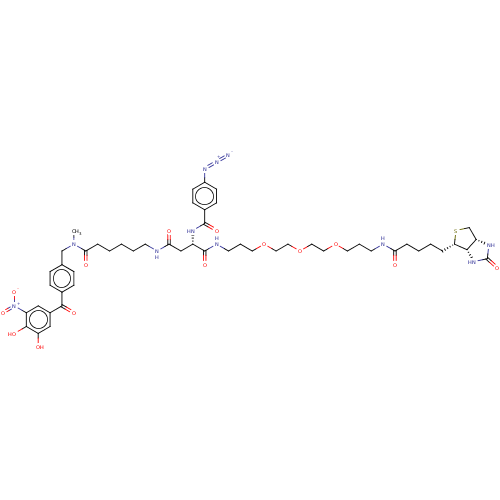 (CHEMBL3819488) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 648 | n/a | n/a | n/a | n/a | n/a | n/a |
Caprotec Bioanalytics GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant His-tagged soluble COMT expressed in Escherichia coli BL21 using aesculetin as substrate after 60 mins by microplate ... | J Med Chem 59: 4664-75 (2016) Article DOI: 10.1021/acs.jmedchem.5b01970 BindingDB Entry DOI: 10.7270/Q2WD42HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50403047 (CHEMBL1231568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant NQO2-mediated mitomycin C metabolism using NADH as cosubstrate incubated for 5 mins prior to NADH additio... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50403047 (CHEMBL1231568) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Displacement of (S)-N4-(4-(3-(2-((4-carbamimidoylphenylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzo[d]imidazole-5-carboxamido)propanamido)butyl)-... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant NQO2-mediated mitomycin C metabolism using NADH as cosubstrate incubated for 5 mins prior to NADH additio... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosyldihydronicotinamide dehydrogenase [quinone] (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
caprotec bioanalytics GmbH Curated by ChEMBL | Assay Description Displacement of (S)-N4-(4-(3-(2-((4-carbamimidoylphenylamino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzo[d]imidazole-5-carboxamido)propanamido)butyl)-... | J Med Chem 55: 3934-44 (2012) Article DOI: 10.1021/jm3001339 BindingDB Entry DOI: 10.7270/Q25T3MNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||