 Found 211 hits with Last Name = 'kucera' and Initial = 't'
Found 211 hits with Last Name = 'kucera' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM412060
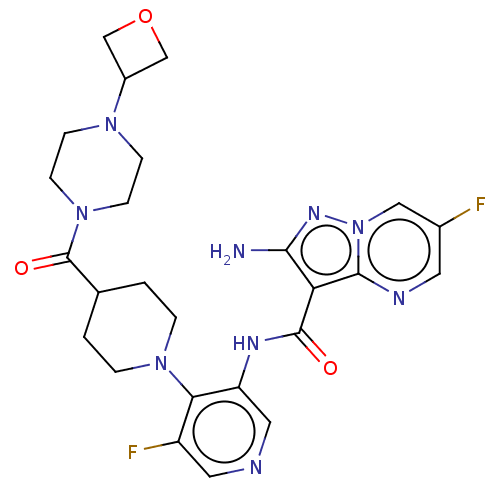
(2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)...)Show SMILES Nc1nn2cc(F)cnc2c1C(=O)Nc1cncc(F)c1N1CCC(CC1)C(=O)N1CCN(CC1)C1COC1 Show InChI InChI=1S/C25H29F2N9O3/c26-16-9-30-23-20(22(28)32-36(23)12-16)24(37)31-19-11-29-10-18(27)21(19)34-3-1-15(2-4-34)25(38)35-7-5-33(6-8-35)17-13-39-14-17/h9-12,15,17H,1-8,13-14H2,(H2,28,32)(H,31,37) | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
UniChem
| Article
PubMed
| <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50555835
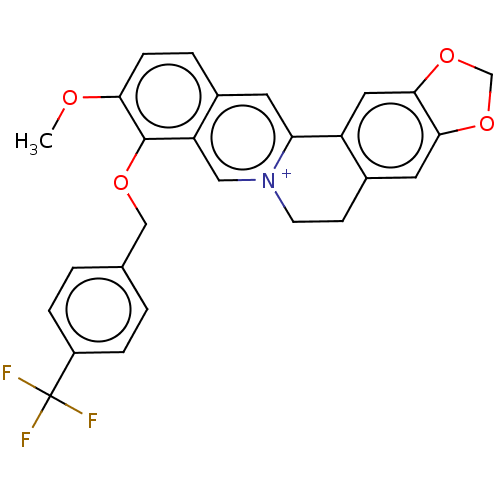
(CHEMBL4752175)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc(cc1)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Noncompetitive inhibition of human acetylcholinesterase assessed as affinity towards free enzyme using acetylthiocholine iodide as substrate measured... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-substrate-inhibitor complex using varying levels of acetylthiocholine iodide as su... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-inhibitor complex using varying levels of acetylthiocholine iodide as substrate pr... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ATR
(Homo sapiens (Human)) | BDBM268079

(2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...)Show SMILES C[C@@H]1COCCN1c1cc(-c2ccnn2C)c2ccnc(-c3ccn[nH]3)c2n1 |r| | PDB
Reactome pathway
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114580
BindingDB Entry DOI: 10.7270/Q261149W |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837
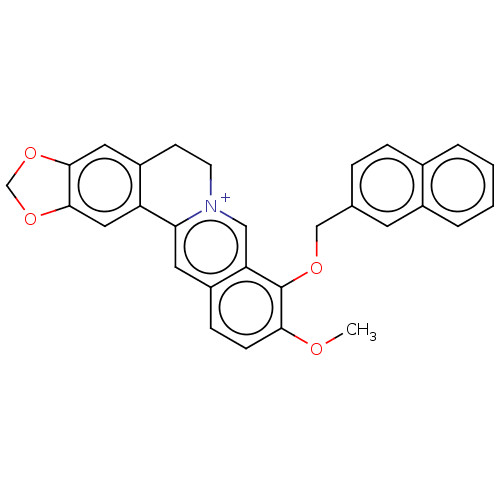
(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards free enzyme using butyrylthiocholine iodide as substrate measured by Ell... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50571189
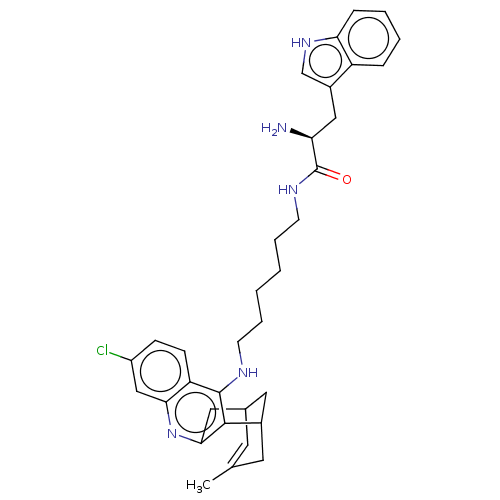
(CHEMBL4856400)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non competitive type inhibition of human AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by double recipr... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50555837

(CHEMBL4747577)Show SMILES [Br-].COc1ccc2cc3-c4cc5OCOc5cc4CC[n+]3cc2c1OCc1ccc2ccccc2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Mixed inhibition of human butyrylcholinesterase assessed as affinity towards enzyme-substrate complex using butyrylthiocholine iodide as substrate me... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112593
BindingDB Entry DOI: 10.7270/Q2HH6PQD |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50508727

((+)-Aromoline | CHEMBL508781)Show SMILES [H][C@@]12Cc3ccc(O)c(Oc4ccc(C[C@]5([H])N(C)CCc6cc(OC)c(O)c(Oc7cc1c(CCN2C)cc7OC)c56)cc4)c3 |r| Show InChI InChI=1S/C36H38N2O6/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)16-22-7-10-29(39)30(17-22)43-25-8-5-21(6-9-25)15-28-34-24(12-14-38(28)2)19-33(42-4)35(40)36(34)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human BuChE assessed as enzyme-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analys... |
J Nat Prod 82: 239-248 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00592
BindingDB Entry DOI: 10.7270/Q2NZ8BXF |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50508727

((+)-Aromoline | CHEMBL508781)Show SMILES [H][C@@]12Cc3ccc(O)c(Oc4ccc(C[C@]5([H])N(C)CCc6cc(OC)c(O)c(Oc7cc1c(CCN2C)cc7OC)c56)cc4)c3 |r| Show InChI InChI=1S/C36H38N2O6/c1-37-13-11-23-18-31(41-3)32-20-26(23)27(37)16-22-7-10-29(39)30(17-22)43-25-8-5-21(6-9-25)15-28-34-24(12-14-38(28)2)19-33(42-4)35(40)36(34)44-32/h5-10,17-20,27-28,39-40H,11-16H2,1-4H3/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of human BuChE assessed as enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrate by Lineweaver-Burk p... |
J Nat Prod 82: 239-248 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00592
BindingDB Entry DOI: 10.7270/Q2NZ8BXF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Homo sapiens (Human)) | BDBM50038879
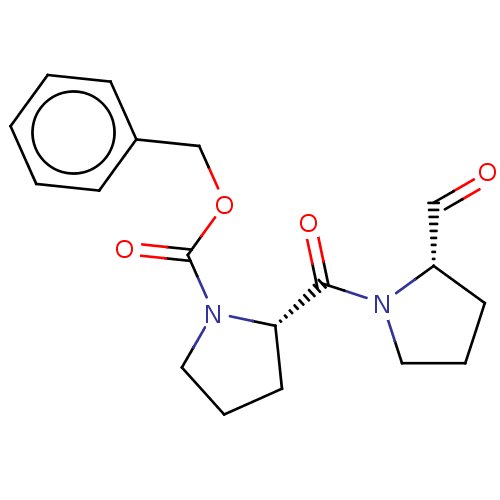
((S)-2-(2-Formyl-pyrrolidine-1-carbonyl)-pyrrolidin...)Show SMILES O=C[C@@H]1CCCN1C(=O)[C@@H]1CCCN1C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C18H22N2O4/c21-12-15-8-4-10-19(15)17(22)16-9-5-11-20(16)18(23)24-13-14-6-2-1-3-7-14/h1-3,6-7,12,15-16H,4-5,8-11,13H2/t15-,16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of POP (unknown origin) using (Z)-Gly-Pro-p-nitroanilide as substrate preincubated for 5 mins followed by substrate addition and measured ... |
J Nat Prod 82: 239-248 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00592
BindingDB Entry DOI: 10.7270/Q2NZ8BXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50523387

(CHEMBL4556281)Show SMILES Cl.Cl.N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C30H36ClN5O.2ClH/c31-21-13-14-24-28(18-21)36-27-12-6-4-10-23(27)29(24)33-15-7-1-2-8-16-34-30(37)25(32)17-20-19-35-26-11-5-3-9-22(20)26;;/h3,5,9,11,13-14,18-19,25,35H,1-2,4,6-8,10,12,15-17,32H2,(H,33,36)(H,34,37);2*1H/t25-;;/m0../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50523387

(CHEMBL4556281)Show SMILES Cl.Cl.N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCCCCCCNc1c2CCCCc2nc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C30H36ClN5O.2ClH/c31-21-13-14-24-28(18-21)36-27-12-6-4-10-23(27)29(24)33-15-7-1-2-8-16-34-30(37)25(32)17-20-19-35-26-11-5-3-9-22(20)26;;/h3,5,9,11,13-14,18-19,25,35H,1-2,4,6-8,10,12,15-17,32H2,(H,33,36)(H,34,37);2*1H/t25-;;/m0../s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50571190
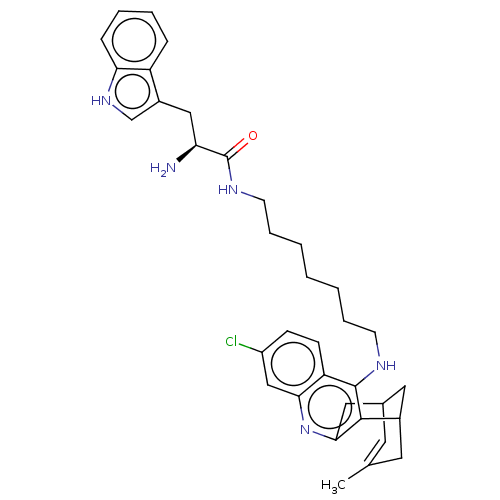
(CHEMBL4878434)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50571189

(CHEMBL4856400)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50571188
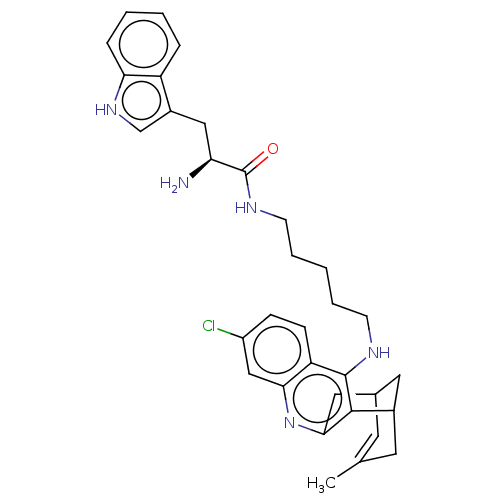
(CHEMBL4874244)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50199522

((+)-huperzine A | (+-)-HA | (-)-1-Amino-13-ethylid...)Show SMILES C\C=C1/[C@@H]2Cc3[nH]c(=O)ccc3[C@@]1(N)CC(C)=C2 |r,c:18,THB:1:2:14.15.17:5.11.4| Show InChI InChI=1S/C15H18N2O/c1-3-11-10-6-9(2)8-15(11,16)12-4-5-14(18)17-13(12)7-10/h3-6,10H,7-8,16H2,1-2H3,(H,17,18)/b11-3+/t10-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method |
J Nat Prod 82: 239-248 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00592
BindingDB Entry DOI: 10.7270/Q2NZ8BXF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50571189

(CHEMBL4856400)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458457
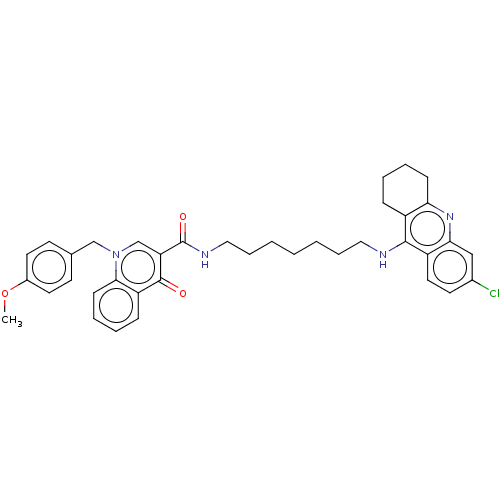
(CHEMBL4213591)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C38H41ClN4O3/c1-46-28-18-15-26(16-19-28)24-43-25-32(37(44)31-12-6-8-14-35(31)43)38(45)41-22-10-4-2-3-9-21-40-36-29-11-5-7-13-33(29)42-34-23-27(39)17-20-30(34)36/h6,8,12,14-20,23,25H,2-5,7,9-11,13,21-22,24H2,1H3,(H,40,42)(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458451
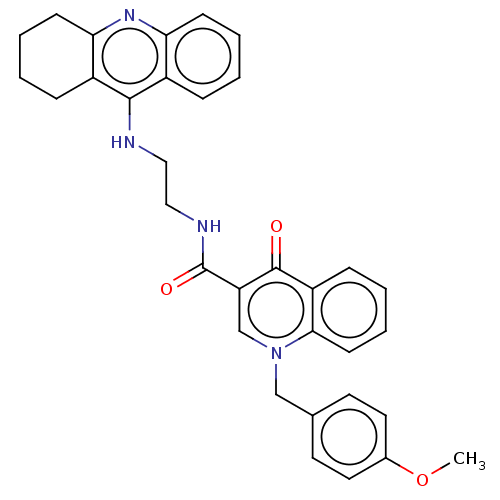
(CHEMBL4205374)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H32N4O3/c1-40-23-16-14-22(15-17-23)20-37-21-27(32(38)26-10-4-7-13-30(26)37)33(39)35-19-18-34-31-24-8-2-5-11-28(24)36-29-12-6-3-9-25(29)31/h2,4-5,7-8,10-11,13-17,21H,3,6,9,12,18-20H2,1H3,(H,34,36)(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50571188

(CHEMBL4874244)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458459
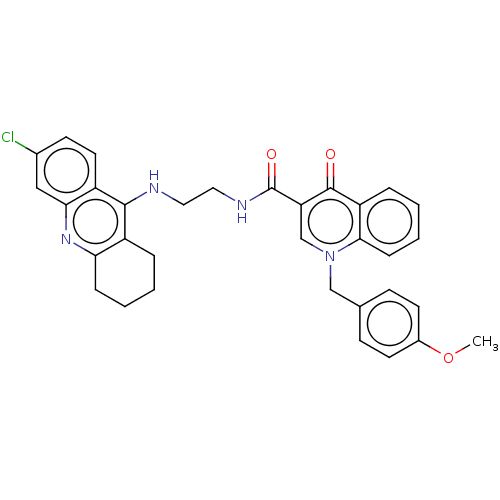
(CHEMBL4215217)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H31ClN4O3/c1-41-23-13-10-21(11-14-23)19-38-20-27(32(39)26-7-3-5-9-30(26)38)33(40)36-17-16-35-31-24-6-2-4-8-28(24)37-29-18-22(34)12-15-25(29)31/h3,5,7,9-15,18,20H,2,4,6,8,16-17,19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458459

(CHEMBL4215217)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H31ClN4O3/c1-41-23-13-10-21(11-14-23)19-38-20-27(32(39)26-7-3-5-9-30(26)38)33(40)36-17-16-35-31-24-6-2-4-8-28(24)37-29-18-22(34)12-15-25(29)31/h3,5,7,9-15,18,20H,2,4,6,8,16-17,19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50571190

(CHEMBL4878434)Show SMILES Cl.Cl.CC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1NCCCCCCCNC(=O)[C@@H](N)Cc1c[nH]c2ccccc12 |r,t:1,TLB:20:9:6:4.8.3,THB:2:3:6:11.10.9| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458448
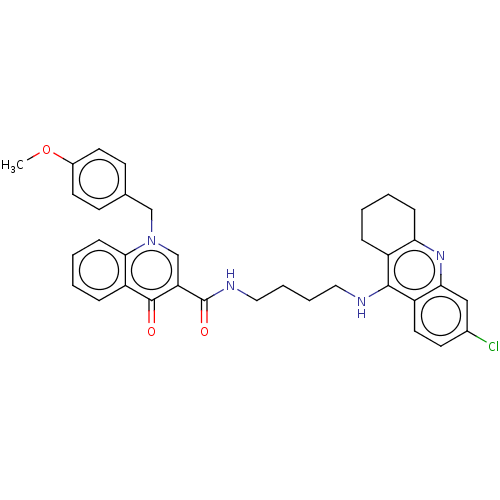
(CHEMBL4215154)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C35H35ClN4O3/c1-43-25-15-12-23(13-16-25)21-40-22-29(34(41)28-9-3-5-11-32(28)40)35(42)38-19-7-6-18-37-33-26-8-2-4-10-30(26)39-31-20-24(36)14-17-27(31)33/h3,5,9,11-17,20,22H,2,4,6-8,10,18-19,21H2,1H3,(H,37,39)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458444

(CHEMBL4217346)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C37H39ClN4O3/c1-45-27-17-14-25(15-18-27)23-42-24-31(36(43)30-11-5-7-13-34(30)42)37(44)40-21-9-3-2-8-20-39-35-28-10-4-6-12-32(28)41-33-22-26(38)16-19-29(33)35/h5,7,11,13-19,22,24H,2-4,6,8-10,12,20-21,23H2,1H3,(H,39,41)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458445
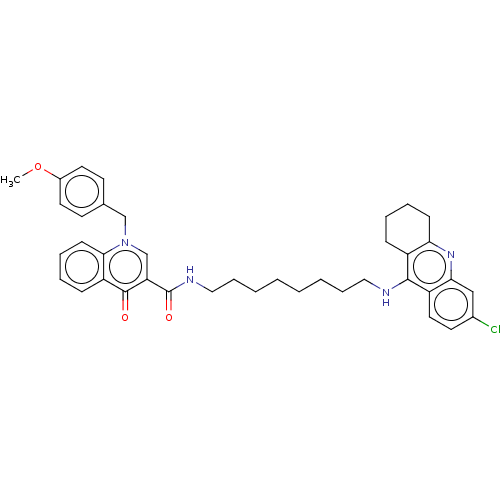
(CHEMBL4203672)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C39H43ClN4O3/c1-47-29-19-16-27(17-20-29)25-44-26-33(38(45)32-13-7-9-15-36(32)44)39(46)42-23-11-5-3-2-4-10-22-41-37-30-12-6-8-14-34(30)43-35-24-28(40)18-21-31(35)37/h7,9,13,15-21,24,26H,2-6,8,10-12,14,22-23,25H2,1H3,(H,41,43)(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458460
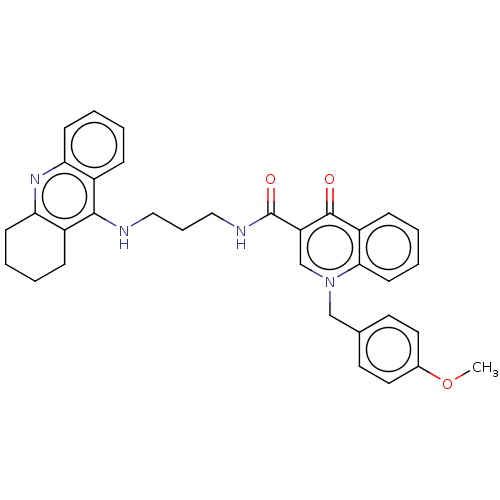
(CHEMBL4214668)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H34N4O3/c1-41-24-17-15-23(16-18-24)21-38-22-28(33(39)27-11-4-7-14-31(27)38)34(40)36-20-8-19-35-32-25-9-2-5-12-29(25)37-30-13-6-3-10-26(30)32/h2,4-5,7,9,11-12,14-18,22H,3,6,8,10,13,19-21H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458447
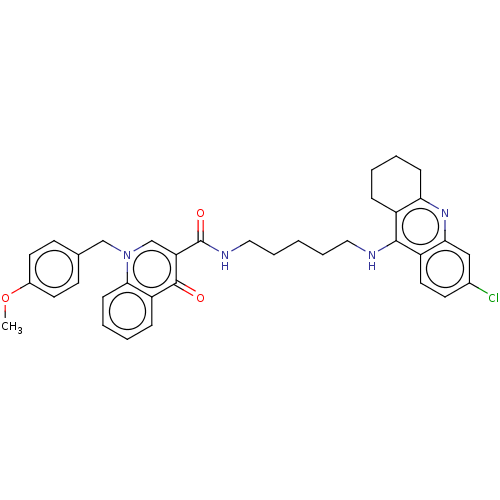
(CHEMBL4217660)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C36H37ClN4O3/c1-44-26-16-13-24(14-17-26)22-41-23-30(35(42)29-10-4-6-12-33(29)41)36(43)39-20-8-2-7-19-38-34-27-9-3-5-11-31(27)40-32-21-25(37)15-18-28(32)34/h4,6,10,12-18,21,23H,2-3,5,7-9,11,19-20,22H2,1H3,(H,38,40)(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458451

(CHEMBL4205374)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H32N4O3/c1-40-23-16-14-22(15-17-23)20-37-21-27(32(38)26-10-4-7-13-30(26)37)33(39)35-19-18-34-31-24-8-2-5-11-28(24)36-29-12-6-3-9-25(29)31/h2,4-5,7-8,10-11,13-17,21H,3,6,9,12,18-20H2,1H3,(H,34,36)(H,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458450
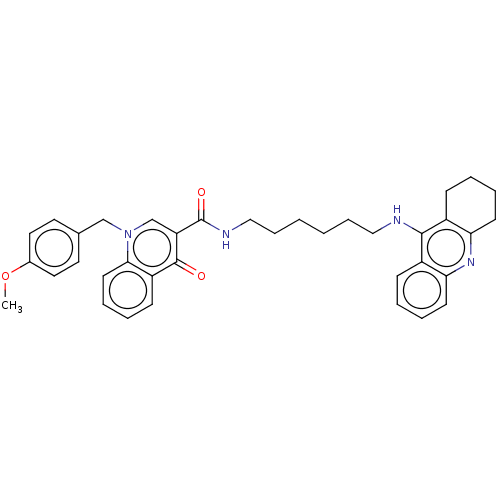
(CHEMBL4206484)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C37H40N4O3/c1-44-27-20-18-26(19-21-27)24-41-25-31(36(42)30-14-6-9-17-34(30)41)37(43)39-23-11-3-2-10-22-38-35-28-12-4-7-15-32(28)40-33-16-8-5-13-29(33)35/h4,6-7,9,12,14-15,17-21,25H,2-3,5,8,10-11,13,16,22-24H2,1H3,(H,38,40)(H,39,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458449
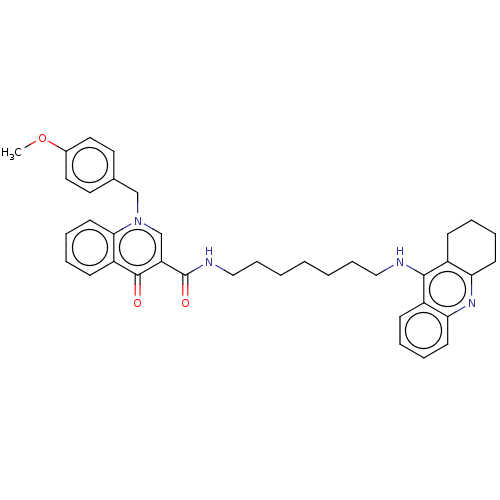
(CHEMBL4214900)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C38H42N4O3/c1-45-28-21-19-27(20-22-28)25-42-26-32(37(43)31-15-7-10-18-35(31)42)38(44)40-24-12-4-2-3-11-23-39-36-29-13-5-8-16-33(29)41-34-17-9-6-14-30(34)36/h5,7-8,10,13,15-16,18-22,26H,2-4,6,9,11-12,14,17,23-25H2,1H3,(H,39,41)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 194 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458450

(CHEMBL4206484)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C37H40N4O3/c1-44-27-20-18-26(19-21-27)24-41-25-31(36(42)30-14-6-9-17-34(30)41)37(43)39-23-11-3-2-10-22-38-35-28-12-4-7-15-32(28)40-33-16-8-5-13-29(33)35/h4,6-7,9,12,14-15,17-21,25H,2-3,5,8,10-11,13,16,22-24H2,1H3,(H,38,40)(H,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458461
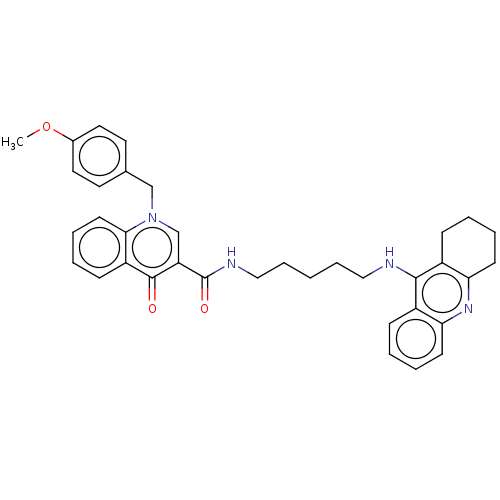
(CHEMBL4202784)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C36H38N4O3/c1-43-26-19-17-25(18-20-26)23-40-24-30(35(41)29-13-5-8-16-33(29)40)36(42)38-22-10-2-9-21-37-34-27-11-3-6-14-31(27)39-32-15-7-4-12-28(32)34/h3,5-6,8,11,13-14,16-20,24H,2,4,7,9-10,12,15,21-23H2,1H3,(H,37,39)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 358 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128100
BindingDB Entry DOI: 10.7270/Q2WD44BG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458443
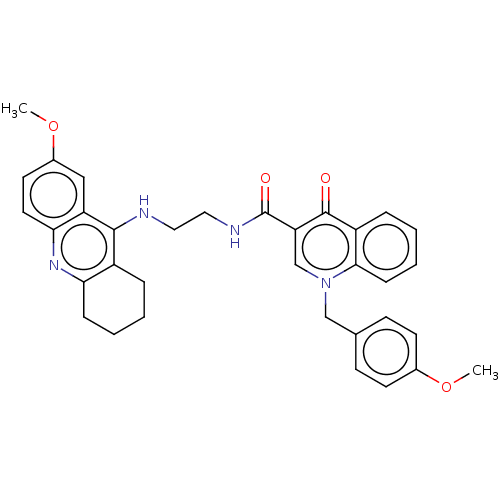
(CHEMBL4212151)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4ccc(OC)cc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H34N4O4/c1-41-23-13-11-22(12-14-23)20-38-21-28(33(39)26-8-4-6-10-31(26)38)34(40)36-18-17-35-32-25-7-3-5-9-29(25)37-30-16-15-24(42-2)19-27(30)32/h4,6,8,10-16,19,21H,3,5,7,9,17-18,20H2,1-2H3,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 408 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50458460

(CHEMBL4214668)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H34N4O3/c1-41-24-17-15-23(16-18-24)21-38-22-28(33(39)27-11-4-7-14-31(27)38)34(40)36-20-8-19-35-32-25-9-2-5-12-29(25)37-30-13-6-3-10-26(30)32/h2,4-5,7,9,11-12,14-18,22H,3,6,8,10,13,19-21H2,1H3,(H,35,37)(H,36,40) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 455 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Antagonist activity at human M1 mAChR expressed in CHO cells assessed as inhibition of oxotremorine M-stimulated calcium influx preincubated for 10 m... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458446
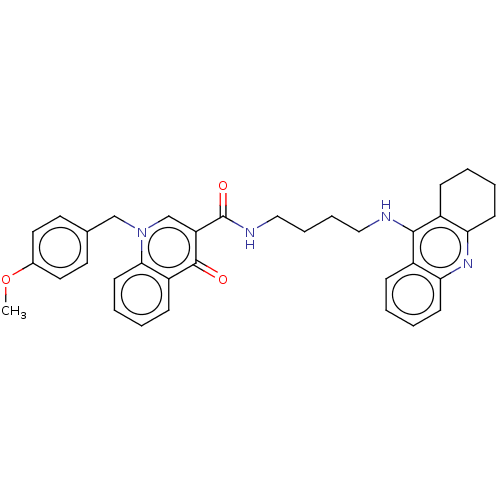
(CHEMBL4209755)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C35H36N4O3/c1-42-25-18-16-24(17-19-25)22-39-23-29(34(40)28-12-4-7-15-32(28)39)35(41)37-21-9-8-20-36-33-26-10-2-5-13-30(26)38-31-14-6-3-11-27(31)33/h2,4-5,7,10,12-13,15-19,23H,3,6,8-9,11,14,20-22H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 491 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50458451

(CHEMBL4205374)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H32N4O3/c1-40-23-16-14-22(15-17-23)20-37-21-27(32(38)26-10-4-7-13-30(26)37)33(39)35-19-18-34-31-24-8-2-5-11-28(24)36-29-12-6-3-9-25(29)31/h2,4-5,7-8,10-11,13-17,21H,3,6,9,12,18-20H2,1H3,(H,34,36)(H,35,39) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Antagonist activity at human M1 mAChR expressed in CHO cells assessed as inhibition of oxotremorine M-stimulated calcium influx preincubated for 10 m... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458460

(CHEMBL4214668)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H34N4O3/c1-41-24-17-15-23(16-18-24)21-38-22-28(33(39)27-11-4-7-14-31(27)38)34(40)36-20-8-19-35-32-25-9-2-5-12-29(25)37-30-13-6-3-10-26(30)32/h2,4-5,7,9,11-12,14-18,22H,3,6,8,10,13,19-21H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50203126
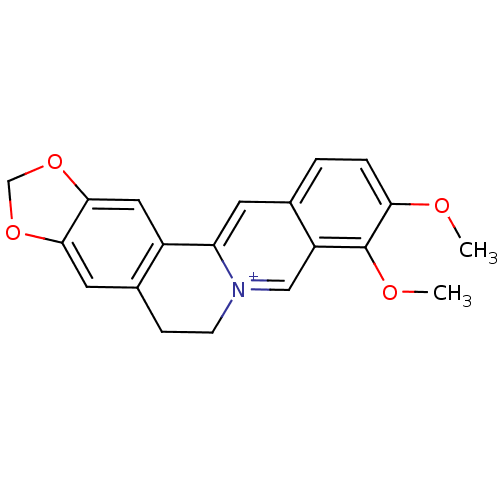
(3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...)Show InChI InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Charles University
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate measured for 1 min by Ellman's method |
J Nat Prod 82: 239-248 (2019)
Article DOI: 10.1021/acs.jnatprod.8b00592
BindingDB Entry DOI: 10.7270/Q2NZ8BXF |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458442
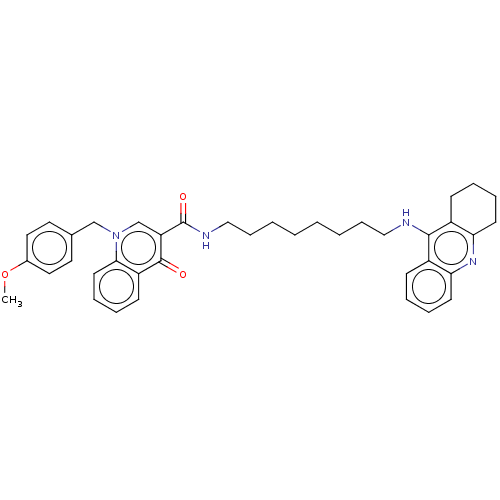
(CHEMBL4204901)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C39H44N4O3/c1-46-29-22-20-28(21-23-29)26-43-27-33(38(44)32-16-8-11-19-36(32)43)39(45)41-25-13-5-3-2-4-12-24-40-37-30-14-6-9-17-34(30)42-35-18-10-7-15-31(35)37/h6,8-9,11,14,16-17,19-23,27H,2-5,7,10,12-13,15,18,24-26H2,1H3,(H,40,42)(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 768 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data