| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Serine/threonine-protein kinase ATR |
|---|
| Ligand | BDBM412060 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2204847 (CHEMBL5117555) |
|---|
| Ki | <0.200000±n/a nM |
|---|
| Citation |  Gorecki, L; Muthna, D; Merdita, S; Andrs, M; Kucera, T; Havelek, R; Muckova, L; Kobrlova, T; Soukup, J; Krupa, P; Prchal, L; Soukup, O; Roh, J; Rezacova, M; Korabecny, J 7-Azaindole, 2,7-diazaindole, and 1H-pyrazole as core structures for novel anticancer agents with potential chemosensitizing properties. Eur J Med Chem240:0 (2022) [PubMed] Article Gorecki, L; Muthna, D; Merdita, S; Andrs, M; Kucera, T; Havelek, R; Muckova, L; Kobrlova, T; Soukup, J; Krupa, P; Prchal, L; Soukup, O; Roh, J; Rezacova, M; Korabecny, J 7-Azaindole, 2,7-diazaindole, and 1H-pyrazole as core structures for novel anticancer agents with potential chemosensitizing properties. Eur J Med Chem240:0 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Serine/threonine-protein kinase ATR |
|---|
| Name: | Serine/threonine-protein kinase ATR |
|---|
| Synonyms: | ATR | ATR_HUMAN | Ataxia telangiectasia and Rad3-related protein (ATR) | FRAP-related protein 1 | FRP1 | Serine-protein kinase ATR |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 301404.58 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | Q13535 |
|---|
| Residue: | 2644 |
|---|
| Sequence: | MGEHGLELASMIPALRELGSATPEEYNTVVQKPRQILCQFIDRILTDVNVVAVELVKKTD
SQPTSVMLLDFIQHIMKSSPLMFVNVSGSHEAKGSCIEFSNWIITRLLRIAATPSCHLLH
KKICEVICSLLFLFKSKSPAIFGVLTKELLQLFEDLVYLHRRNVMGHAVEWPVVMSRFLS
QLDEHMGYLQSAPLQLMSMQNLEFIEVTLLMVLTRIIAIVFFRRQELLLWQIGCVLLEYG
SPKIKSLAISFLTELFQLGGLPAQPASTFFSSFLELLKHLVEMDTDQLKLYEEPLSKLIK
TLFPFEAEAYRNIEPVYLNMLLEKLCVMFEDGVLMRLKSDLLKAALCHLLQYFLKFVPAG
YESALQVRKVYVRNICKALLDVLGIEVDAEYLLGPLYAALKMESMEIIEEIQCQTQQENL
SSNSDGISPKRRRLSSSLNPSKRAPKQTEEIKHVDMNQKSILWSALKQKAESLQISLEYS
GLKNPVIEMLEGIAVVLQLTALCTVHCSHQNMNCRTFKDCQHKSKKKPSVVITWMSLDFY
TKVLKSCRSLLESVQKLDLEATIDKVVKIYDALIYMQVNSSFEDHILEDLCGMLSLPWIY
SHSDDGCLKLTTFAANLLTLSCRISDSYSPQAQSRCVFLLTLFPRRIFLEWRTAVYNWAL
QSSHEVIRASCVSGFFILLQQQNSCNRVPKILIDKVKDDSDIVKKEFASILGQLVCTLHG
MFYLTSSLTEPFSEHGHVDLFCRNLKATSQHECSSSQLKASVCKPFLFLLKKKIPSPVKL
AFIDNLHHLCKHLDFREDETDVKAVLGTLLNLMEDPDKDVRVAFSGNIKHILESLDSEDG
FIKELFVLRMKEAYTHAQISRNNELKDTLILTTGDIGRAAKGDLVPFALLHLLHCLLSKS
ASVSGAAYTEIRALVAAKSVKLQSFFSQYKKPICQFLVESLHSSQMTALPNTPCQNADVR
KQDVAHQREMALNTLSEIANVFDFPDLNRFLTRTLQVLLPDLAAKASPAASALIRTLGKQ
LNVNRREILINNFKYIFSHLVCSCSKDELERALHYLKNETEIELGSLLRQDFQGLHNELL
LRIGEHYQQVFNGLSILASFASSDDPYQGPRDIISPELMADYLQPKLLGILAFFNMQLLS
SSVGIEDKKMALNSLMSLMKLMGPKHVSSVRVKMMTTLRTGLRFKDDFPELCCRAWDCFV
RCLDHACLGSLLSHVIVALLPLIHIQPKETAAIFHYLIIENRDAVQDFLHEIYFLPDHPE
LKKIKAVLQEYRKETSESTDLQTTLQLSMKAIQHENVDVRIHALTSLKETLYKNQEKLIK
YATDSETVEPIISQLVTVLLKGCQDANSQARLLCGECLGELGAIDPGRLDFSTTETQGKD
FTFVTGVEDSSFAYGLLMELTRAYLAYADNSRAQDSAAYAIQELLSIYDCREMETNGPGH
QLWRRFPEHVREILEPHLNTRYKSSQKSTDWSGVKKPIYLSKLGSNFAEWSASWAGYLIT
KVRHDLASKIFTCCSIMMKHDFKVTIYLLPHILVYVLLGCNQEDQQEVYAEIMAVLKHDD
QHTINTQDIASDLCQLSTQTVFSMLDHLTQWARHKFQALKAEKCPHSKSNRNKVDSMVST
VDYEDYQSVTRFLDLIPQDTLAVASFRSKAYTRAVMHFESFITEKKQNIQEHLGFLQKLY
AAMHEPDGVAGVSAIRKAEPSLKEQILEHESLGLLRDATACYDRAIQLEPDQIIHYHGVV
KSMLGLGQLSTVITQVNGVHANRSEWTDELNTYRVEAAWKLSQWDLVENYLAADGKSTTW
SVRLGQLLLSAKKRDITAFYDSLKLVRAEQIVPLSAASFERGSYQRGYEYIVRLHMLCEL
EHSIKPLFQHSPGDSSQEDSLNWVARLEMTQNSYRAKEPILALRRALLSLNKRPDYNEMV
GECWLQSARVARKAGHHQTAYNALLNAGESRLAELYVERAKWLWSKGDVHQALIVLQKGV
ELCFPENETPPEGKNMLIHGRAMLLVGRFMEETANFESNAIMKKYKDVTACLPEWEDGHF
YLAKYYDKLMPMVTDNKMEKQGDLIRYIVLHFGRSLQYGNQFIYQSMPRMLTLWLDYGTK
AYEWEKAGRSDRVQMRNDLGKINKVITEHTNYLAPYQFLTAFSQLISRICHSHDEVFVVL
MEIIAKVFLAYPQQAMWMMTAVSKSSYPMRVNRCKEILNKAIHMKKSLEKFVGDATRLTD
KLLELCNKPVDGSSSTLSMSTHFKMLKKLVEEATFSEILIPLQSVMIPTLPSILGTHANH
ASHEPFPGHWAYIAGFDDMVEILASLQKPKKISLKGSDGKFYIMMCKPKDDLRKDCRLME
FNSLINKCLRKDAESRRRELHIRTYAVIPLNDECGIIEWVNNTAGLRPILTKLYKEKGVY
MTGKELRQCMLPKSAALSEKLKVFREFLLPRHPPIFHEWFLRTFPDPTSWYSSRSAYCRS
TAVMSMVGYILGLGDRHGENILFDSLTGECVHVDFNCLFNKGETFEVPEIVPFRLTHNMV
NGMGPMGTEGLFRRACEVTMRLMRDQREPLMSVLKTFLHDPLVEWSKPVKGHSKAPLNET
GEVVNEKAKTHVLDIEQRLQGVIKTRNRVTGLPLSIEGHVHYLIQEATDENLLCQMYLGW
TPYM
|
|
|
|---|
| BDBM412060 |
|---|
| n/a |
|---|
| Name | BDBM412060 |
|---|
| Synonyms: | 2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)piperazine-1-carbonyl]-1-piperidyl]-3-pyridyl]pyrazolo pyrimidine-3-carboxamide | US10392391, Compound I-G-32 | US10787452, Compound I-G-32 | US11117900, Compound I-G-32 | US11370798, Cmpd. # I-G-32 | US20230271963, Compound I-G-32 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C25H29F2N9O3 |
|---|
| Mol. Mass. | 541.5531 |
|---|
| SMILES | Nc1nn2cc(F)cnc2c1C(=O)Nc1cncc(F)c1N1CCC(CC1)C(=O)N1CCN(CC1)C1COC1 |
|---|
| Structure | 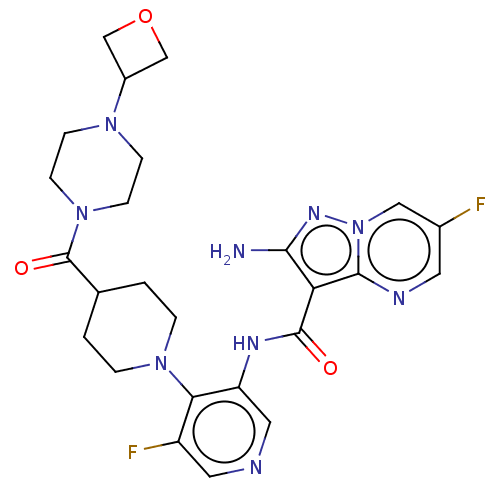
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Gorecki, L; Muthna, D; Merdita, S; Andrs, M; Kucera, T; Havelek, R; Muckova, L; Kobrlova, T; Soukup, J; Krupa, P; Prchal, L; Soukup, O; Roh, J; Rezacova, M; Korabecny, J 7-Azaindole, 2,7-diazaindole, and 1H-pyrazole as core structures for novel anticancer agents with potential chemosensitizing properties. Eur J Med Chem240:0 (2022) [PubMed] Article
Gorecki, L; Muthna, D; Merdita, S; Andrs, M; Kucera, T; Havelek, R; Muckova, L; Kobrlova, T; Soukup, J; Krupa, P; Prchal, L; Soukup, O; Roh, J; Rezacova, M; Korabecny, J 7-Azaindole, 2,7-diazaindole, and 1H-pyrazole as core structures for novel anticancer agents with potential chemosensitizing properties. Eur J Med Chem240:0 (2022) [PubMed] Article