

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM412060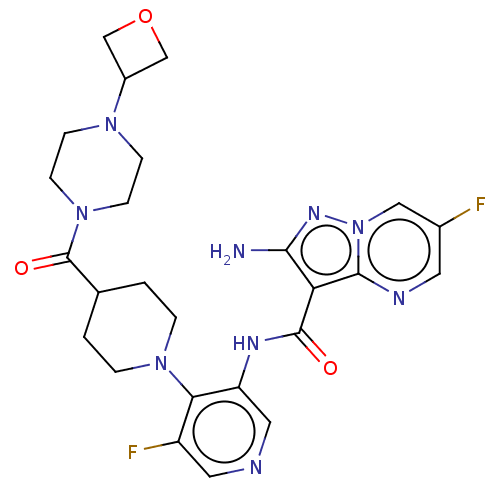 (2-amino-6-fluoro-N-[5-fluoro-4-[4-[4-(oxetan-3-yl)...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE UniChem | Article PubMed | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114580 BindingDB Entry DOI: 10.7270/Q261149W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50458458 (CHEMBL4210729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Mixed type inhibition of human erythrocyte AChE assessed as enzyme-substrate-inhibitor complex using varying levels of acetylthiocholine iodide as su... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50458458 (CHEMBL4210729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Mixed type inhibition of human erythrocyte AChE assessed as enzyme-inhibitor complex using varying levels of acetylthiocholine iodide as substrate pr... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268079 (2-[(3R)-3-methylmorpholin-4-yl]-4-(1-methyl-1H-pyr...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114580 BindingDB Entry DOI: 10.7270/Q261149W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571189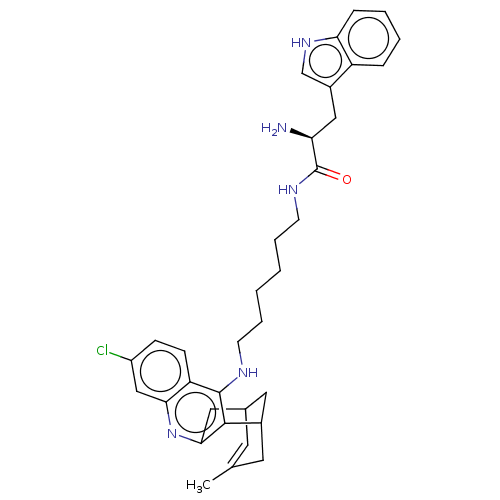 (CHEMBL4856400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive type inhibition of human AChE assessed as inhibition constant using varying levels of acetylthiocholine as substrate by double recipr... | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50133473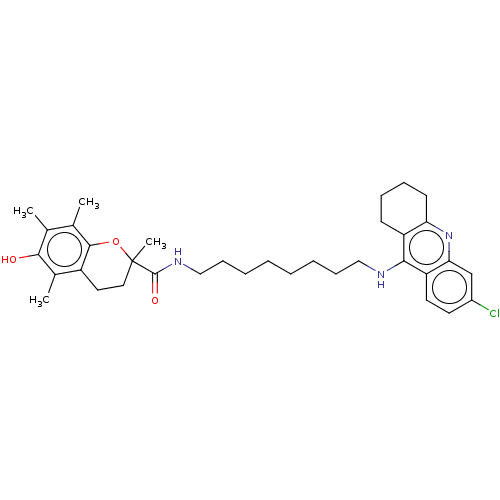 (CHEMBL3632994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50028685 (CHEMBL3356536) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523394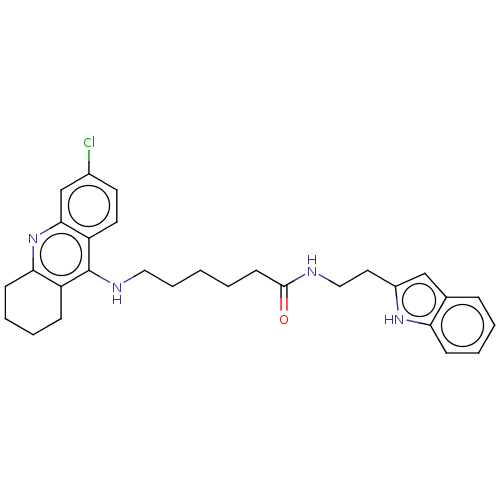 (CHEMBL4448188) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50099602 (CHEMBL3343931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523391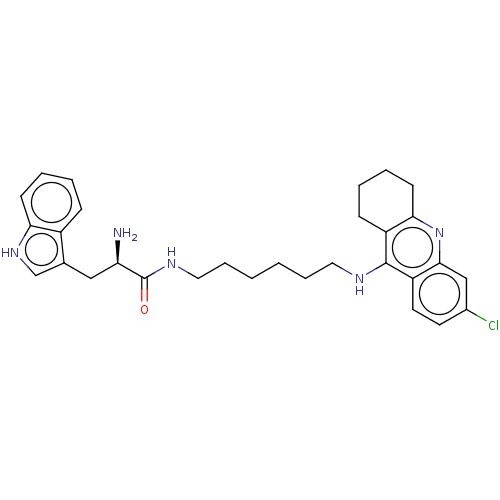 (CHEMBL4449083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523377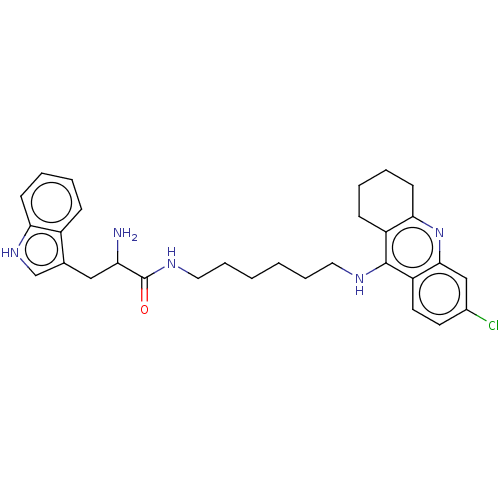 (CHEMBL4559593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523376 (CHEMBL4589980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523387 (CHEMBL4556281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571189 (CHEMBL4856400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571190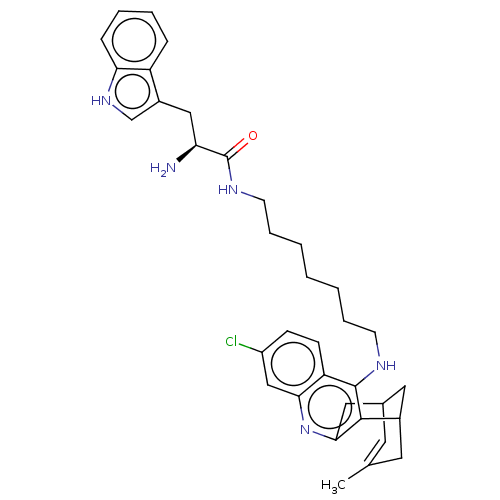 (CHEMBL4878434) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523377 (CHEMBL4559593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523375 (CHEMBL4555818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50133449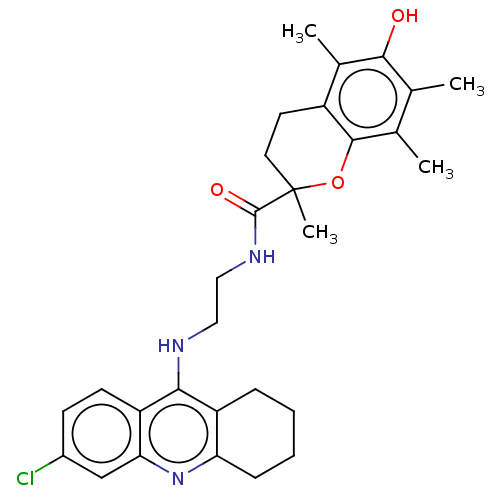 (CHEMBL3632988) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 min... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50571188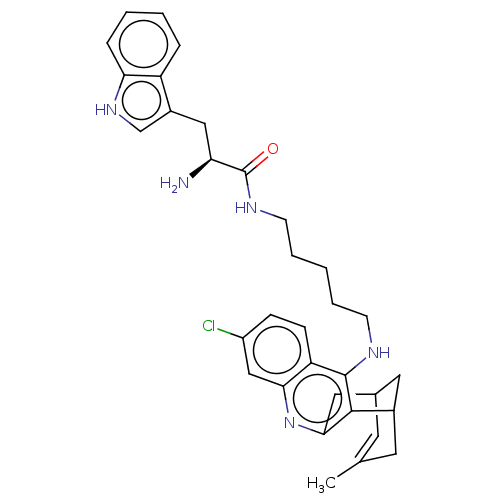 (CHEMBL4874244) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human AChE using acetylthiocholine as substrate by DTNB-reagent based Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523388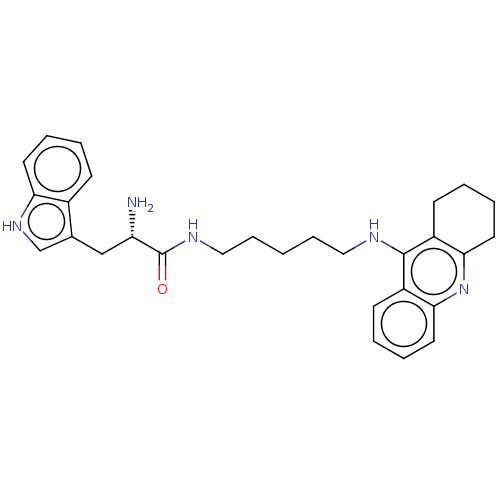 (CHEMBL4556575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523389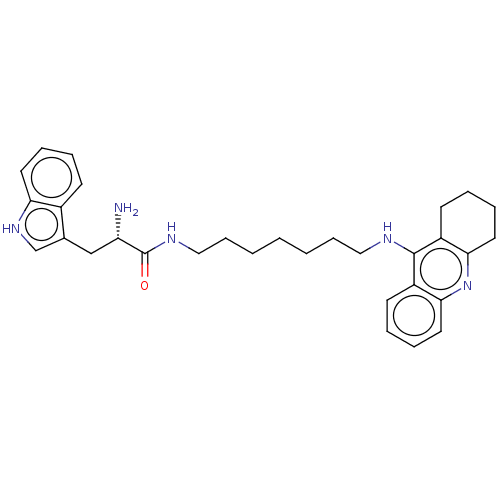 (CHEMBL4559477) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523383 (CHEMBL4438120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50458458 (CHEMBL4210729) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571189 (CHEMBL4856400) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523393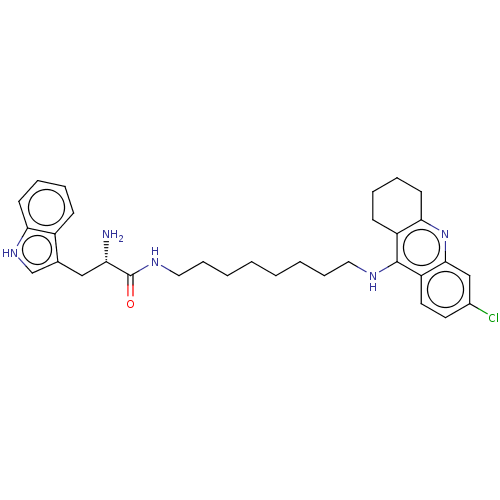 (CHEMBL4466307) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-A preincubated for 5 mins followed by addition of Kynuramine substrate and measured after 30 mins by plate reader method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113112 BindingDB Entry DOI: 10.7270/Q2P55S8J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50570221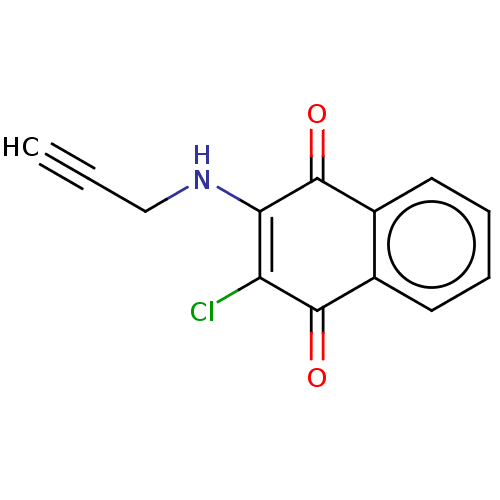 (CHEMBL4866391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human MAO-B preincubated for 5 mins followed by addition of Kynuramine substrate and measured after 30 mins by plate reader method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113112 BindingDB Entry DOI: 10.7270/Q2P55S8J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523375 (CHEMBL4555818) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50458457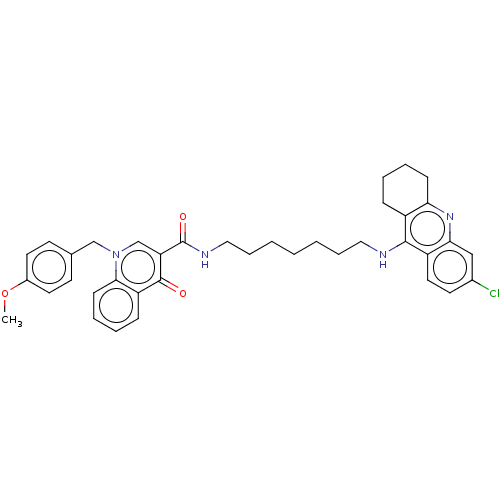 (CHEMBL4213591) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523379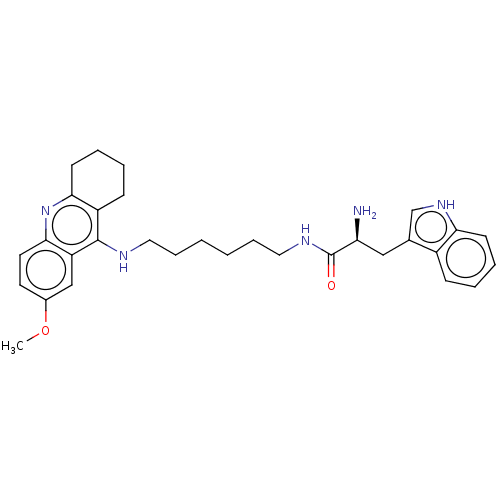 (CHEMBL4467355) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50265253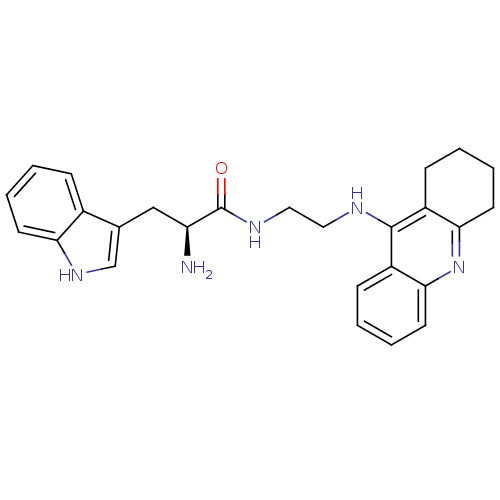 ((S)-2-amino-3-(1H-indol-3-yl)-N-(2-(1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50458451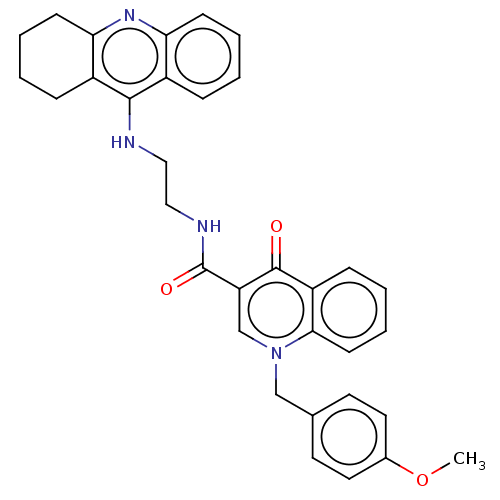 (CHEMBL4205374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50073117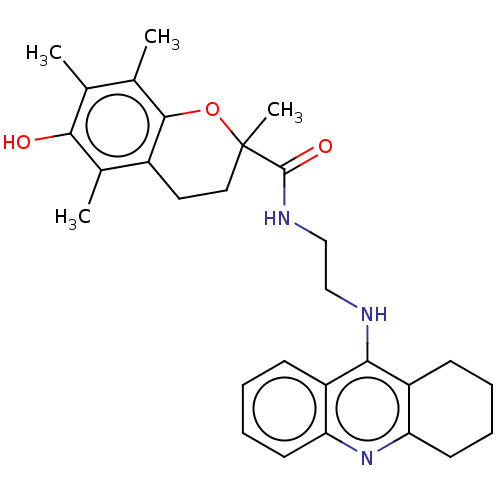 (CHEMBL3410951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human plasmatic BChE using butyrylthiocholine as substrate preincubated for 5 mins followed by substrate addition measured every 2 mins... | J Med Chem 58: 8985-9003 (2015) Article DOI: 10.1021/acs.jmedchem.5b01325 BindingDB Entry DOI: 10.7270/Q29P33GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523373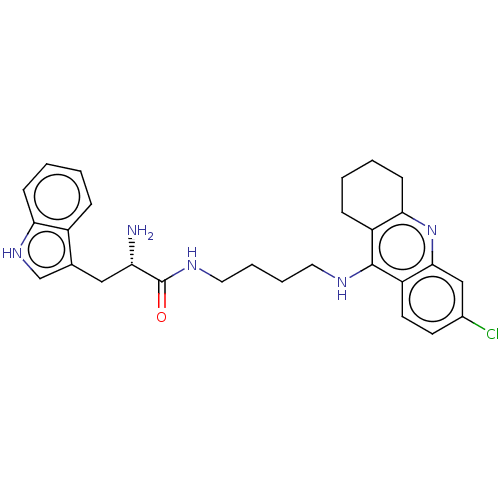 (CHEMBL4552440) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523371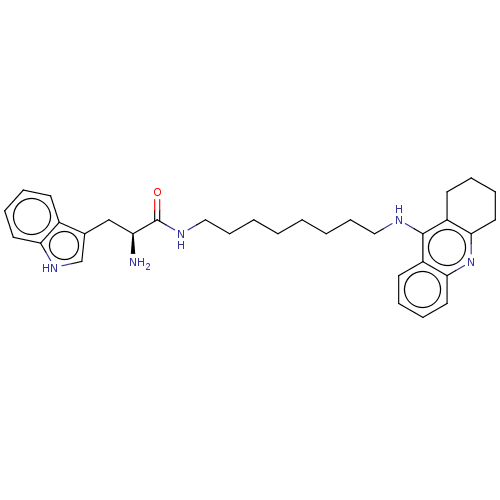 (CHEMBL4456435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50571188 (CHEMBL4874244) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human BuChE using butyrylthiocholine as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128100 BindingDB Entry DOI: 10.7270/Q2WD44BG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523386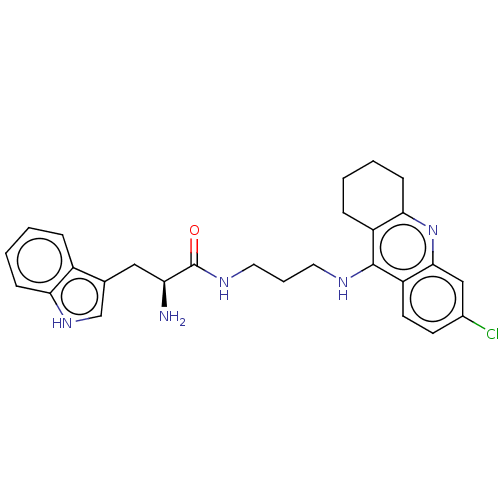 (CHEMBL4541558) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50523382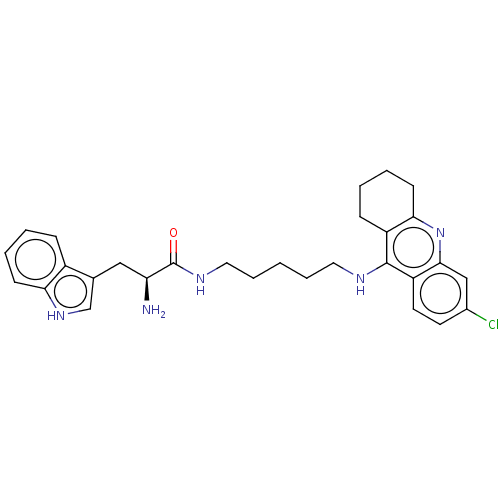 (CHEMBL4563209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50458459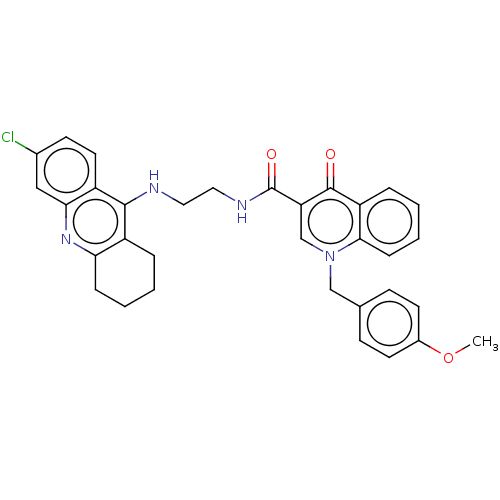 (CHEMBL4215217) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... | Eur J Med Chem 150: 292-306 (2018) Article DOI: 10.1016/j.ejmech.2018.02.083 BindingDB Entry DOI: 10.7270/Q2W95CT0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523371 (CHEMBL4456435) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523382 (CHEMBL4563209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hradec Kralove Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition and measured ... | Eur J Med Chem 168: 491-514 (2019) Article DOI: 10.1016/j.ejmech.2019.02.021 BindingDB Entry DOI: 10.7270/Q2RV0S3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 249 total ) | Next | Last >> |