 Found 252 hits with Last Name = 'kuroha' and Initial = 'm'
Found 252 hits with Last Name = 'kuroha' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50314948
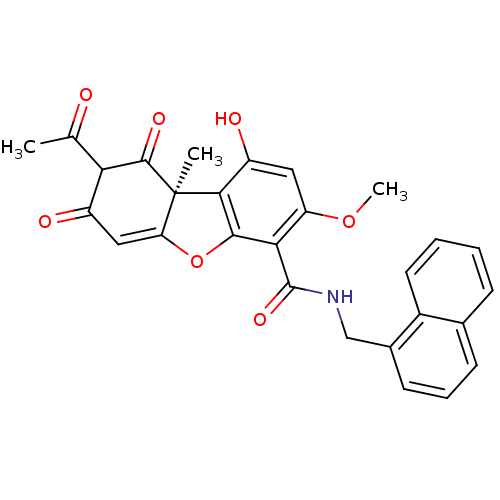
((S)-8-Acetyl-6,9-dihydroxy-3-methoxy-6b-methyl-1,7...)Show SMILES COc1cc(O)c2c(OC3=CC(=O)C(C(C)=O)C(=O)[C@@]23C)c1C(=O)NCc1cccc2ccccc12 |r,t:9| Show InChI InChI=1S/C28H23NO7/c1-14(30)22-18(31)12-21-28(2,26(22)33)24-19(32)11-20(35-3)23(25(24)36-21)27(34)29-13-16-9-6-8-15-7-4-5-10-17(15)16/h4-12,22,32H,13H2,1-3H3,(H,29,34)/t22?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to PPARgamma |
Bioorg Med Chem Lett 20: 2095-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.073
BindingDB Entry DOI: 10.7270/Q2RX9D21 |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50600305
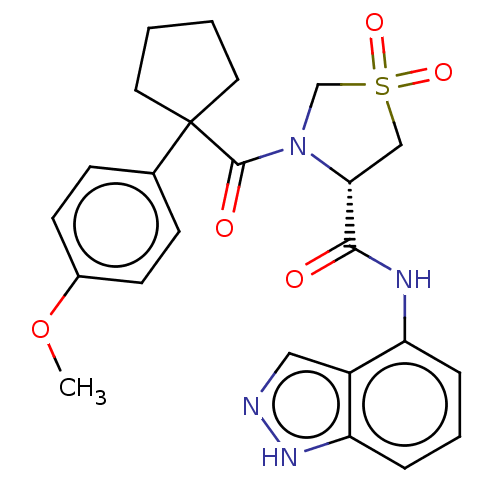
(CHEMBL5193672)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CS(=O)(=O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C-like 1
(Candida albicans) | BDBM50256734
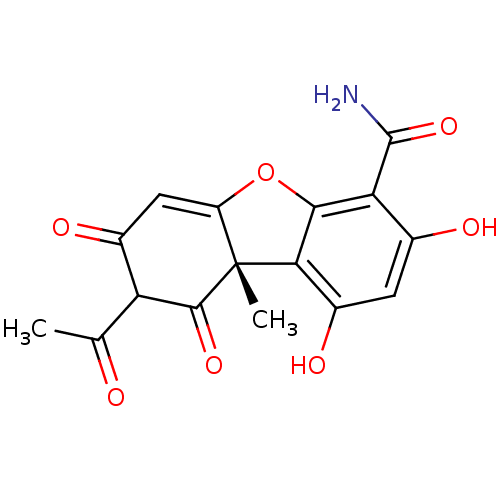
((-)-Cercosporamide | CHEMBL475816)Show SMILES CC(=O)C1C(=O)C=C2Oc3c(c(O)cc(O)c3C(N)=O)[C@]2(C)C1=O |r,t:6| Show InChI InChI=1S/C16H13NO7/c1-5(18)10-7(20)4-9-16(2,14(10)22)12-8(21)3-6(19)11(15(17)23)13(12)24-9/h3-4,10,19,21H,1-2H3,(H2,17,23)/t10?,16-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of Candida albicans Pkc1 expressed in insect Sf9 cells by scintillation proximity assay |
Bioorg Med Chem Lett 19: 724-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.035
BindingDB Entry DOI: 10.7270/Q2H41R95 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600302

(CHEMBL5193437)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@H](F)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600297
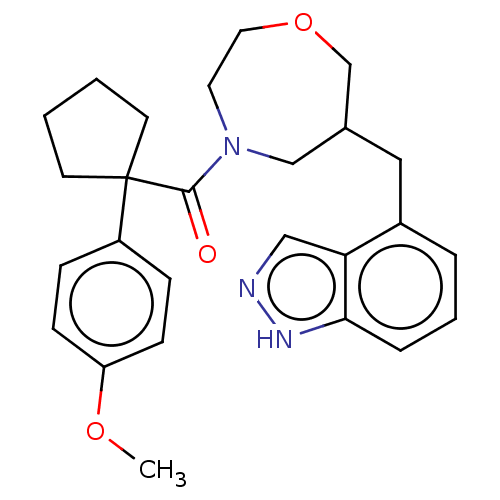
(CHEMBL5174606)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600297

(CHEMBL5174606)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600296
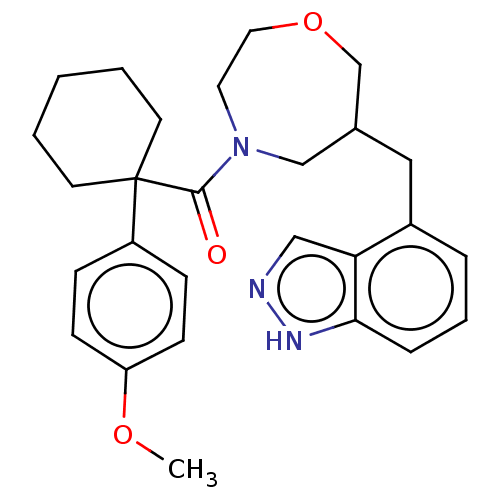
(CHEMBL5177811)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600305

(CHEMBL5193672)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CS(=O)(=O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600300

(CHEMBL5184238)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600301
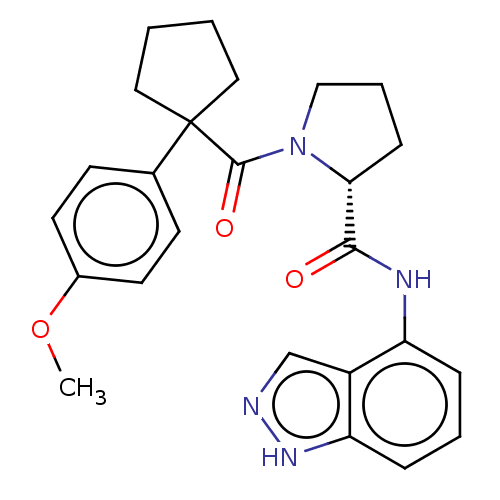
(CHEMBL5178312)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600298
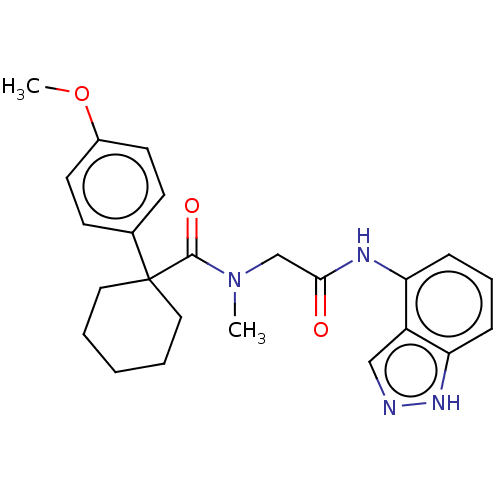
(CHEMBL5187549)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N(C)CC(=O)Nc1cccc2[nH]ncc12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600299
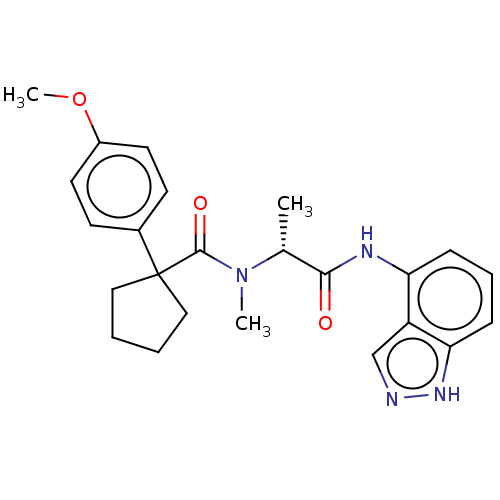
(CHEMBL5191305)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N(C)[C@H](C)C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600304

(CHEMBL5207535)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@H](O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50256734

((-)-Cercosporamide | CHEMBL475816)Show SMILES CC(=O)C1C(=O)C=C2Oc3c(c(O)cc(O)c3C(N)=O)[C@]2(C)C1=O |r,t:6| Show InChI InChI=1S/C16H13NO7/c1-5(18)10-7(20)4-9-16(2,14(10)22)12-8(21)3-6(19)11(15(17)23)13(12)24-9/h3-4,10,19,21H,1-2H3,(H2,17,23)/t10?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PKCbeta by scintillation proximity assay |
Bioorg Med Chem Lett 19: 724-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.035
BindingDB Entry DOI: 10.7270/Q2H41R95 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600297

(CHEMBL5174606)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600297

(CHEMBL5174606)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600300

(CHEMBL5184238)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600305

(CHEMBL5193672)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CS(=O)(=O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600302

(CHEMBL5193437)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@H](F)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600295
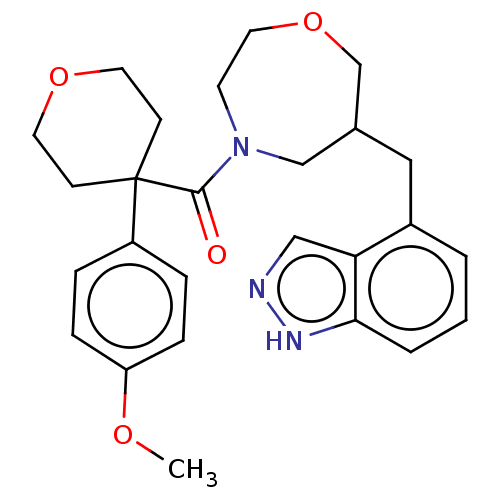
(CHEMBL5209027)Show SMILES COc1ccc(cc1)C1(CCOCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600296

(CHEMBL5177811)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50314948

((S)-8-Acetyl-6,9-dihydroxy-3-methoxy-6b-methyl-1,7...)Show SMILES COc1cc(O)c2c(OC3=CC(=O)C(C(C)=O)C(=O)[C@@]23C)c1C(=O)NCc1cccc2ccccc12 |r,t:9| Show InChI InChI=1S/C28H23NO7/c1-14(30)22-18(31)12-21-28(2,26(22)33)24-19(32)11-20(35-3)23(25(24)36-21)27(34)29-13-16-9-6-8-15-7-4-5-10-17(15)16/h4-12,22,32H,13H2,1-3H3,(H,29,34)/t22?,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma assessed as transcriptional activity |
Bioorg Med Chem Lett 20: 2095-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.073
BindingDB Entry DOI: 10.7270/Q2RX9D21 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600301

(CHEMBL5178312)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600303

(CHEMBL5180471)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@@H](O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600298

(CHEMBL5187549)Show SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N(C)CC(=O)Nc1cccc2[nH]ncc12 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50256734

((-)-Cercosporamide | CHEMBL475816)Show SMILES CC(=O)C1C(=O)C=C2Oc3c(c(O)cc(O)c3C(N)=O)[C@]2(C)C1=O |r,t:6| Show InChI InChI=1S/C16H13NO7/c1-5(18)10-7(20)4-9-16(2,14(10)22)12-8(21)3-6(19)11(15(17)23)13(12)24-9/h3-4,10,19,21H,1-2H3,(H2,17,23)/t10?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PKCalpha by scintillation proximity assay |
Bioorg Med Chem Lett 19: 724-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.035
BindingDB Entry DOI: 10.7270/Q2H41R95 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600304

(CHEMBL5207535)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@H](O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50579801
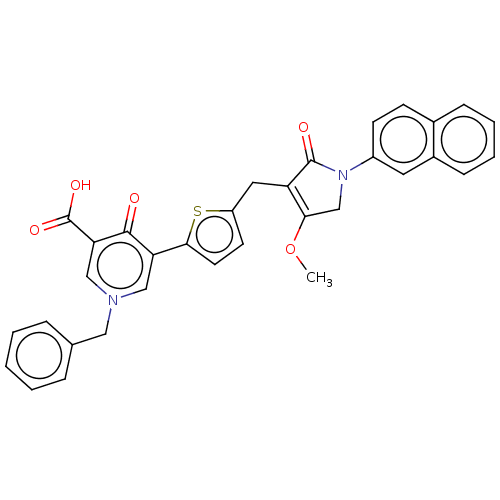
(CHEMBL5088992)Show SMILES COC1=C(Cc2ccc(s2)-c2cn(Cc3ccccc3)cc(C(O)=O)c2=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600303

(CHEMBL5180471)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1C[C@@H](O)C[C@@H]1C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600295

(CHEMBL5209027)Show SMILES COc1ccc(cc1)C1(CCOCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579797
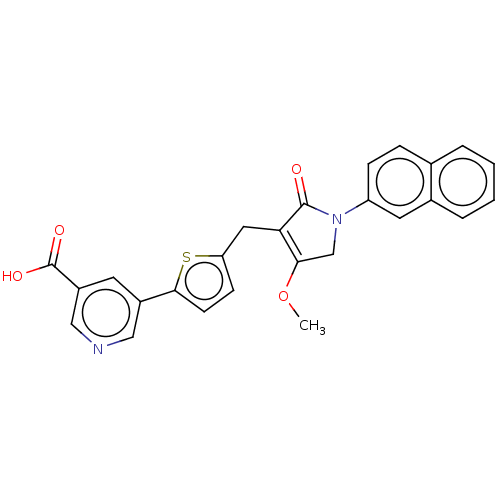
(CHEMBL5071269)Show SMILES COC1=C(Cc2ccc(s2)-c2cncc(c2)C(O)=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579796

(CHEMBL5087034)Show SMILES COC1=C(Cc2ccc(s2)-c2cccc(c2)C(O)=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
CREB-binding protein
(Homo sapiens (Human)) | BDBM50579798
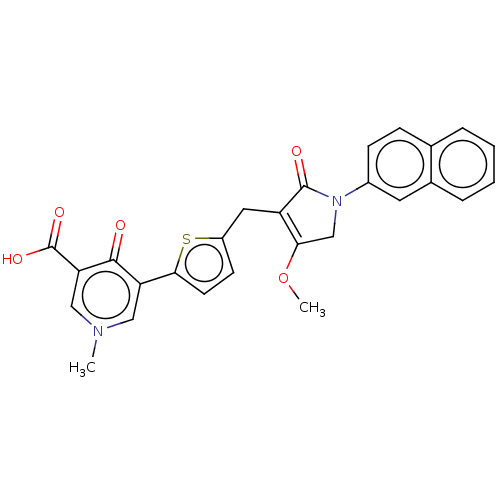
(CHEMBL5080324)Show SMILES COC1=C(Cc2ccc(s2)-c2cn(C)cc(C(O)=O)c2=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CBP (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579801

(CHEMBL5088992)Show SMILES COC1=C(Cc2ccc(s2)-c2cn(Cc3ccccc3)cc(C(O)=O)c2=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600299

(CHEMBL5191305)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N(C)[C@H](C)C(=O)Nc1cccc2[nH]ncc12 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50256734

((-)-Cercosporamide | CHEMBL475816)Show SMILES CC(=O)C1C(=O)C=C2Oc3c(c(O)cc(O)c3C(N)=O)[C@]2(C)C1=O |r,t:6| Show InChI InChI=1S/C16H13NO7/c1-5(18)10-7(20)4-9-16(2,14(10)22)12-8(21)3-6(19)11(15(17)23)13(12)24-9/h3-4,10,19,21H,1-2H3,(H2,17,23)/t10?,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human PKCgamma by scintillation proximity assay |
Bioorg Med Chem Lett 19: 724-6 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.035
BindingDB Entry DOI: 10.7270/Q2H41R95 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT7
(Homo sapiens) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579799
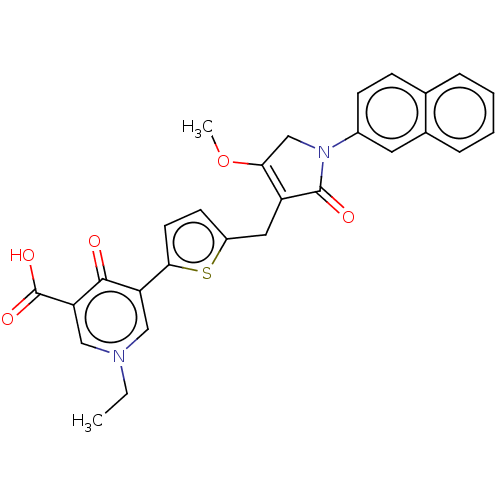
(CHEMBL5093074)Show SMILES CCn1cc(C(O)=O)c(=O)c(c1)-c1ccc(CC2=C(CN(C2=O)c2ccc3ccccc3c2)OC)s1 |t:18| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579798

(CHEMBL5080324)Show SMILES COC1=C(Cc2ccc(s2)-c2cn(C)cc(C(O)=O)c2=O)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579800

(CHEMBL5083461)Show SMILES COC1=C(Cc2ccc(s2)-c2cn(cc(C(O)=O)c2=O)C(C)C)C(=O)N(C1)c1ccc2ccccc2c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50314950

((S)-8-Acetyl-6,9-dihydroxy-3-methoxy-6b-methyl-1,7...)Show SMILES COc1cc(O)c2c(OC3=CC(=O)C(C(C)=O)C(=O)[C@@]23C)c1C(=O)NCc1ccco1 |r,t:9| Show InChI InChI=1S/C22H19NO8/c1-10(24)16-12(25)8-15-22(2,20(16)27)18-13(26)7-14(29-3)17(19(18)31-15)21(28)23-9-11-5-4-6-30-11/h4-8,16,26H,9H2,1-3H3,(H,23,28)/t16?,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of PPARgamma assessed as transcriptional activity |
Bioorg Med Chem Lett 20: 2095-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.073
BindingDB Entry DOI: 10.7270/Q2RX9D21 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579794
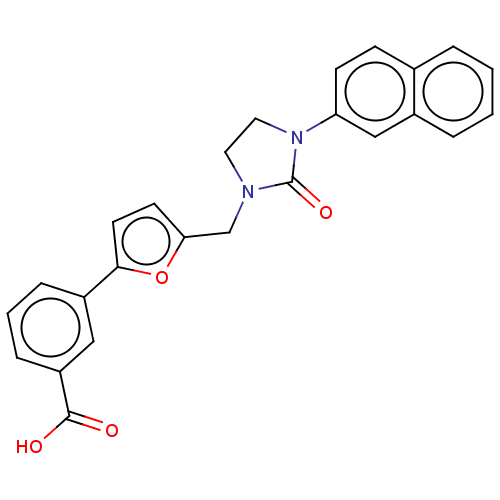
(CHEMBL5086865)Show SMILES OC(=O)c1cccc(c1)-c1ccc(CN2CCN(C2=O)c2ccc3ccccc3c2)o1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579795
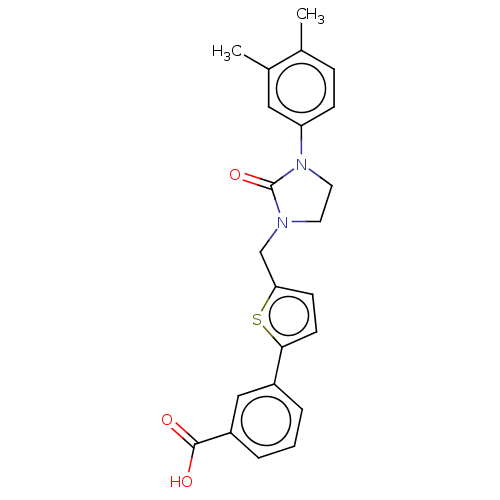
(CHEMBL5089215)Show SMILES Cc1ccc(cc1C)N1CCN(Cc2ccc(s2)-c2cccc(c2)C(O)=O)C1=O | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT6B
(Homo sapiens) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50600297

(CHEMBL5174606)Show SMILES COc1ccc(cc1)C1(CCCC1)C(=O)N1CCOCC(Cc2cccc3[nH]ncc23)C1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579792

(CHEMBL5086573)Show SMILES COC1=C(Cc2ccc(o2)-c2cccc(c2)C(O)=O)C(=O)N(C1)c1ccc(C)c(C)c1 |c:2| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579793
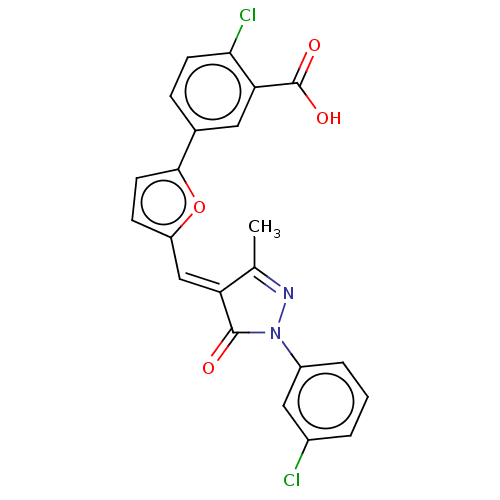
(CHEMBL5074998)Show SMILES CC1=NN(C(=O)\C1=C\c1ccc(o1)-c1ccc(Cl)c(c1)C(O)=O)c1cccc(Cl)c1 |t:1| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT5
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50579791
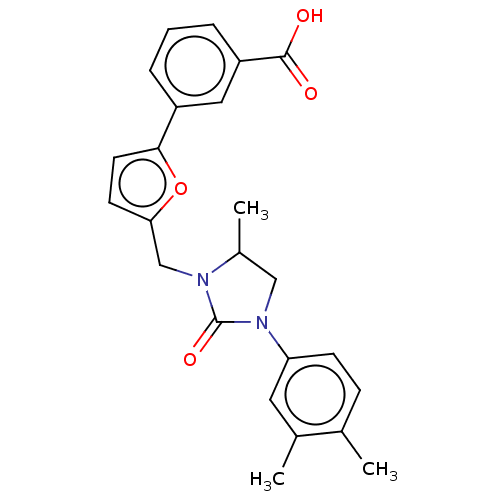
(CHEMBL5069865)Show SMILES CC1CN(C(=O)N1Cc1ccc(o1)-c1cccc(c1)C(O)=O)c1ccc(C)c(C)c1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EP300 (unknown origin) by AlphaLISA immunodetection assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128358
BindingDB Entry DOI: 10.7270/Q23R0XQQ |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase KAT2B
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128726
BindingDB Entry DOI: 10.7270/Q27W6H8T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data