 Found 32 hits with Last Name = 'lacour' and Initial = 'c'
Found 32 hits with Last Name = 'lacour' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(BOVINE) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 1122-30 (2002)
Article DOI: 10.1124/jpet.300.3.1122
BindingDB Entry DOI: 10.7270/Q20V8BBW |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282260

(7-Butyl-8-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCCCC1=NC2(CCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C26H30N6O/c1-2-3-10-23-27-26(15-6-7-16-26)17-24(33)32(23)18-19-11-13-20(14-12-19)21-8-4-5-9-22(21)25-28-30-31-29-25/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282261
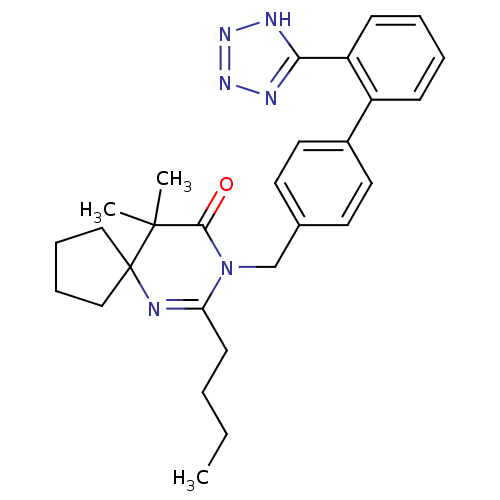
(7-Butyl-10,10-dimethyl-8-[2'-(2H-tetrazol-5-yl)-bi...)Show SMILES CCCCC1=NC2(CCCC2)C(C)(C)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-4-5-12-24-29-28(17-8-9-18-28)27(2,3)26(35)34(24)19-20-13-15-21(16-14-20)22-10-6-7-11-23(22)25-30-32-33-31-25/h6-7,10-11,13-16H,4-5,8-9,12,17-19H2,1-3H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282270

(4'-(2-Butyl-4-cyclopentyl-4-methyl-5-oxo-4,5-dihyd...)Show SMILES CCCCC1=NC(C)(C2CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C27H32N2O3/c1-3-4-13-24-28-27(2,21-9-5-6-10-21)26(32)29(24)18-19-14-16-20(17-15-19)22-11-7-8-12-23(22)25(30)31/h7-8,11-12,14-17,21H,3-6,9-10,13,18H2,1-2H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282264
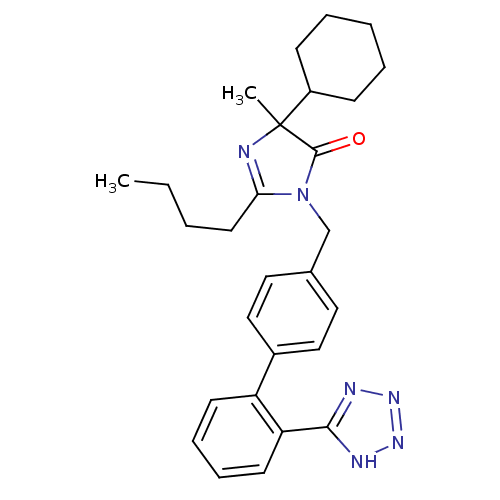
(2-Butyl-5-cyclohexyl-5-methyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282264

(2-Butyl-5-cyclohexyl-5-methyl-3-[2'-(1H-tetrazol-5...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C28H34N6O/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(35)34(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26-30-32-33-31-26/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282267
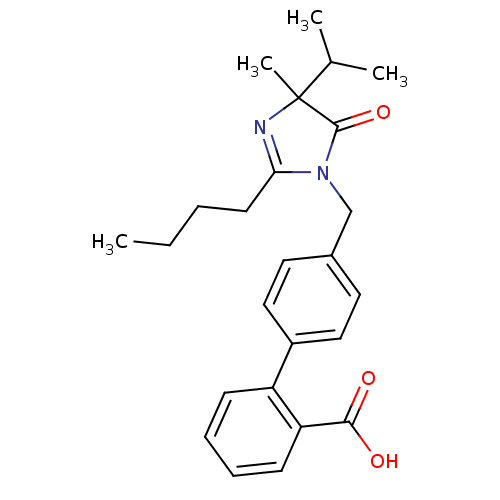
(4'-(2-Butyl-4-isopropyl-4-methyl-5-oxo-4,5-dihydro...)Show SMILES CCCCC1=NC(C)(C(C)C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H30N2O3/c1-5-6-11-22-26-25(4,17(2)3)24(30)27(22)16-18-12-14-19(15-13-18)20-9-7-8-10-21(20)23(28)29/h7-10,12-15,17H,5-6,11,16H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282263
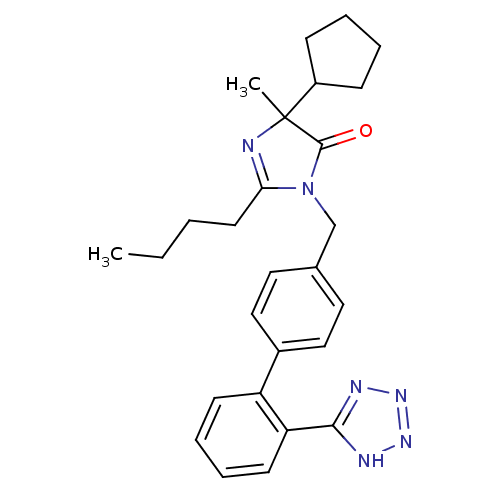
(2-Butyl-5-cyclopentyl-5-methyl-3-[2'-(2H-tetrazol-...)Show SMILES CCCCC1=NC(C)(C2CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C27H32N6O/c1-3-4-13-24-28-27(2,21-9-5-6-10-21)26(34)33(24)18-19-14-16-20(17-15-19)22-11-7-8-12-23(22)25-29-31-32-30-25/h7-8,11-12,14-17,21H,3-6,9-10,13,18H2,1-2H3,(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282262
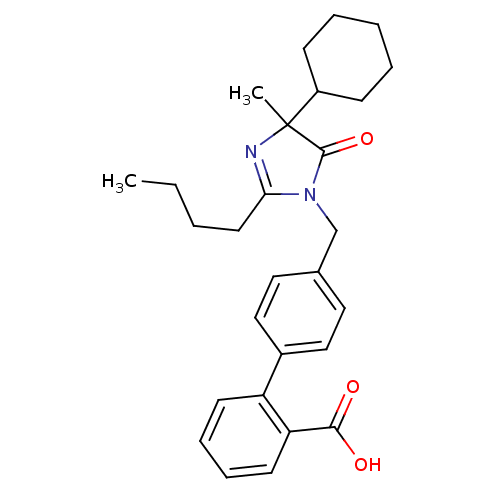
(4'-(2-Butyl-4-cyclohexyl-4-methyl-5-oxo-4,5-dihydr...)Show SMILES CCCCC1=NC(C)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C28H34N2O3/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(33)30(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26(31)32/h8-9,12-13,15-18,22H,3-7,10-11,14,19H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282268
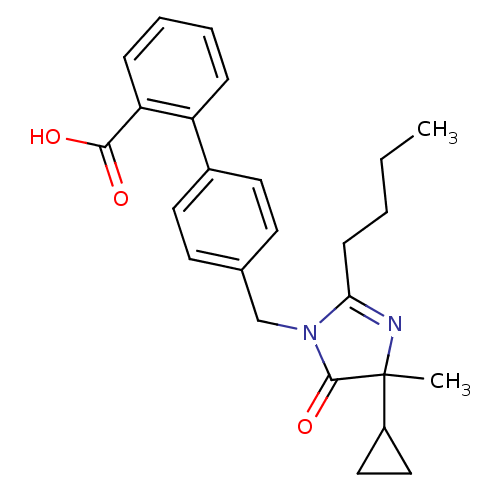
(4'-(2-Butyl-4-cyclopropyl-4-methyl-5-oxo-4,5-dihyd...)Show SMILES CCCCC1=NC(C)(C2CC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H28N2O3/c1-3-4-9-22-26-25(2,19-14-15-19)24(30)27(22)16-17-10-12-18(13-11-17)20-7-5-6-8-21(20)23(28)29/h5-8,10-13,19H,3-4,9,14-16H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282265
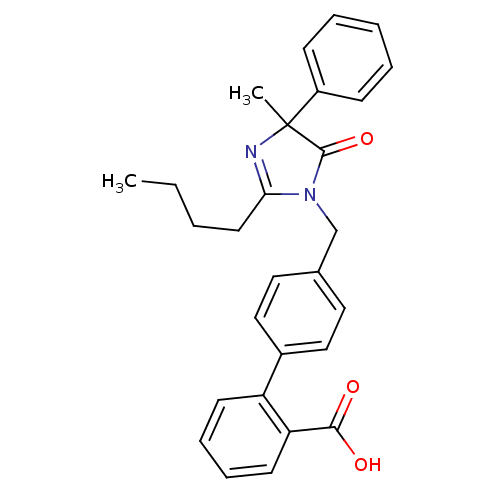
(4'-(2-Butyl-4-methyl-5-oxo-4-phenyl-4,5-dihydro-im...)Show SMILES CCCCC1=NC(C)(C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O)c1ccccc1 |t:4| Show InChI InChI=1S/C28H28N2O3/c1-3-4-14-25-29-28(2,22-10-6-5-7-11-22)27(33)30(25)19-20-15-17-21(18-16-20)23-12-8-9-13-24(23)26(31)32/h5-13,15-18H,3-4,14,19H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042244
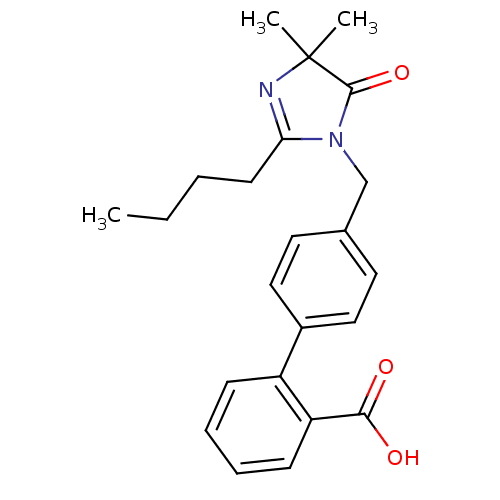
(4'-(2-Butyl-4,4-dimethyl-5-oxo-4,5-dihydro-imidazo...)Show SMILES CCCCC1=NC(C)(C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C23H26N2O3/c1-4-5-10-20-24-23(2,3)22(28)25(20)15-16-11-13-17(14-12-16)18-8-6-7-9-19(18)21(26)27/h6-9,11-14H,4-5,10,15H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
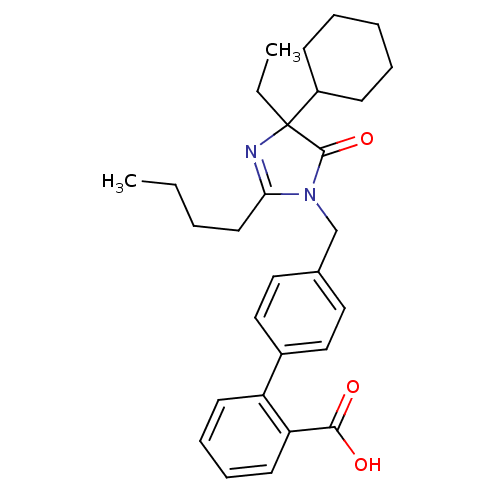
Type-1 angiotensin II receptor B
(RAT) | BDBM50282269
(4'-(2-Butyl-4-cyclohexyl-4-ethyl-5-oxo-4,5-dihydro...)Show SMILES CCCCC1=NC(CC)(C2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H36N2O3/c1-3-5-15-26-30-29(4-2,23-11-7-6-8-12-23)28(34)31(26)20-21-16-18-22(19-17-21)24-13-9-10-14-25(24)27(32)33/h9-10,13-14,16-19,23H,3-8,11-12,15,20H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
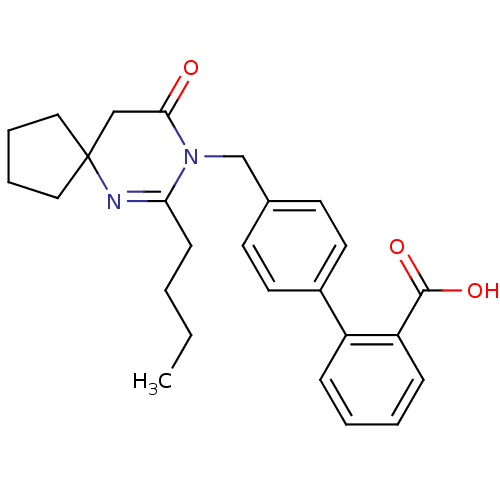
Type-1 angiotensin II receptor B
(RAT) | BDBM50282257
(4'-(7-Butyl-9-oxo-6,8-diaza-spiro[4.5]dec-6-en-8-y...)Show SMILES CCCCC1=NC2(CCCC2)CC(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C26H30N2O3/c1-2-3-10-23-27-26(15-6-7-16-26)17-24(29)28(23)18-19-11-13-20(14-12-19)21-8-4-5-9-22(21)25(30)31/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
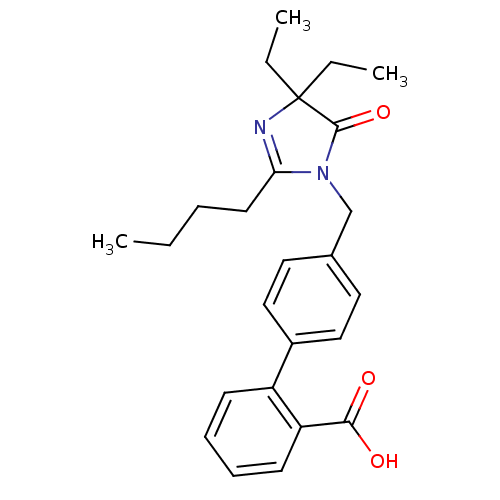
Type-1 angiotensin II receptor B
(RAT) | BDBM50042240
(4'-(2-Butyl-4,4-diethyl-5-oxo-4,5-dihydro-imidazol...)Show SMILES CCCCC1=NC(CC)(CC)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C25H30N2O3/c1-4-7-12-22-26-25(5-2,6-3)24(30)27(22)17-18-13-15-19(16-14-18)20-10-8-9-11-21(20)23(28)29/h8-11,13-16H,4-7,12,17H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
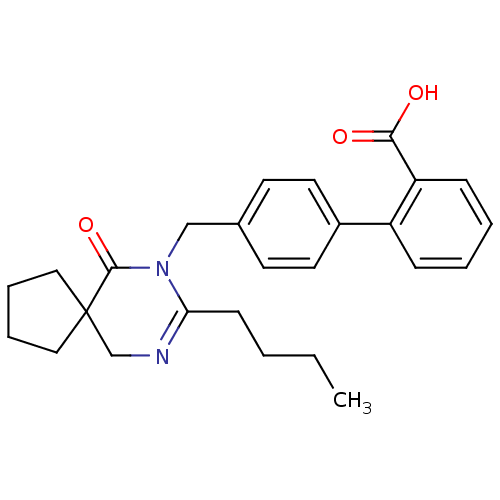
Type-1 angiotensin II receptor B
(RAT) | BDBM50282258
(4'-(8-Butyl-6-oxo-7,9-diaza-spiro[4.5]dec-8-en-7-y...)Show SMILES CCCCC1=NCC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C26H30N2O3/c1-2-3-10-23-27-18-26(15-6-7-16-26)25(31)28(23)17-19-11-13-20(14-12-19)21-8-4-5-9-22(21)24(29)30/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042239
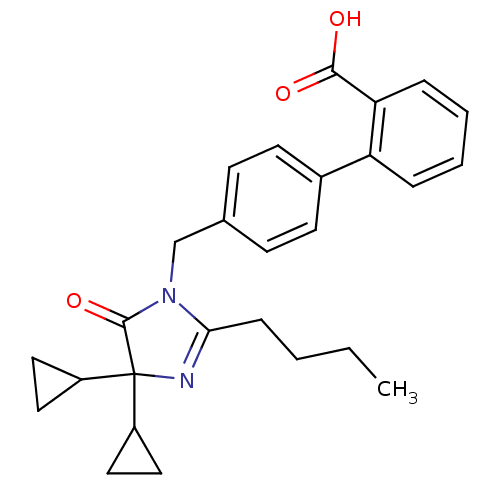
(4'-(2-Butyl-4,4-dicyclopropyl-5-oxo-4,5-dihydro-im...)Show SMILES CCCCC1=NC(C2CC2)(C2CC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C27H30N2O3/c1-2-3-8-24-28-27(20-13-14-20,21-15-16-21)26(32)29(24)17-18-9-11-19(12-10-18)22-6-4-5-7-23(22)25(30)31/h4-7,9-12,20-21H,2-3,8,13-17H2,1H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282266
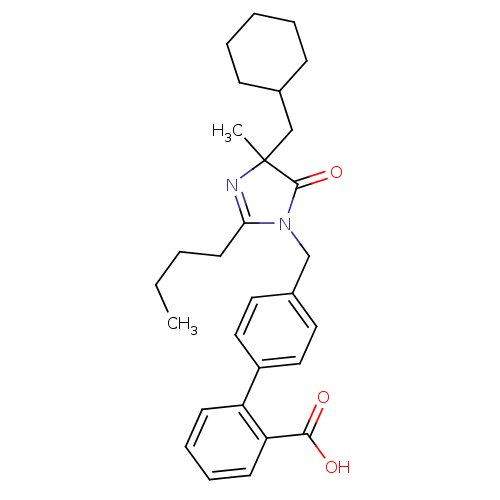
(4'-(2-Butyl-4-cyclohexylmethyl-4-methyl-5-oxo-4,5-...)Show SMILES CCCCC1=NC(C)(CC2CCCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H36N2O3/c1-3-4-14-26-30-29(2,19-21-10-6-5-7-11-21)28(34)31(26)20-22-15-17-23(18-16-22)24-12-8-9-13-25(24)27(32)33/h8-9,12-13,15-18,21H,3-7,10-11,14,19-20H2,1-2H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282271
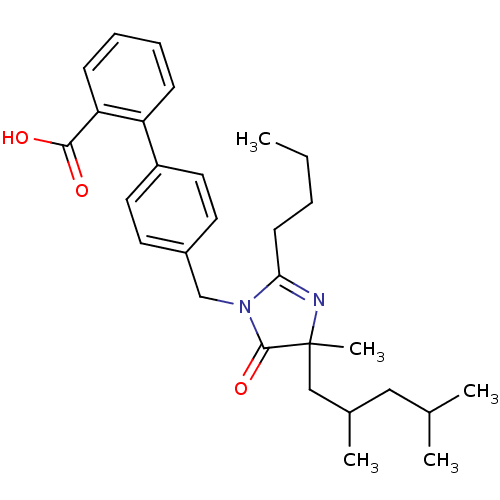
(4'-[2-Butyl-4-(2,4-dimethyl-pentyl)-4-methyl-5-oxo...)Show SMILES CCCCC1=NC(C)(CC(C)CC(C)C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H38N2O3/c1-6-7-12-26-30-29(5,18-21(4)17-20(2)3)28(34)31(26)19-22-13-15-23(16-14-22)24-10-8-9-11-25(24)27(32)33/h8-11,13-16,20-21H,6-7,12,17-19H2,1-5H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50282259
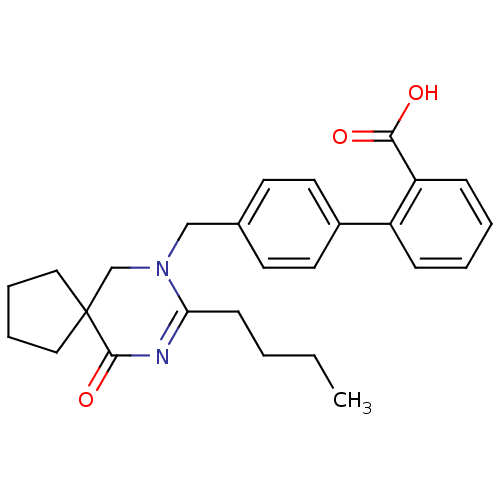
(4'-(8-Butyl-10-oxo-7,9-diaza-spiro[4.5]dec-8-en-7-...)Show SMILES CCCCC1=NC(=O)C2(CCCC2)CN1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C26H30N2O3/c1-2-3-10-23-27-25(31)26(15-6-7-16-26)18-28(23)17-19-11-13-20(14-12-19)21-8-4-5-9-22(21)24(29)30/h4-5,8-9,11-14H,2-3,6-7,10,15-18H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific binding of [125I]-AII to Angiotensin II receptor, type 1 from rat liver membrane |
Bioorg Med Chem Lett 4: 157-162 (1994)
Article DOI: 10.1016/S0960-894X(01)81139-2
BindingDB Entry DOI: 10.7270/Q2QN66QV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042222
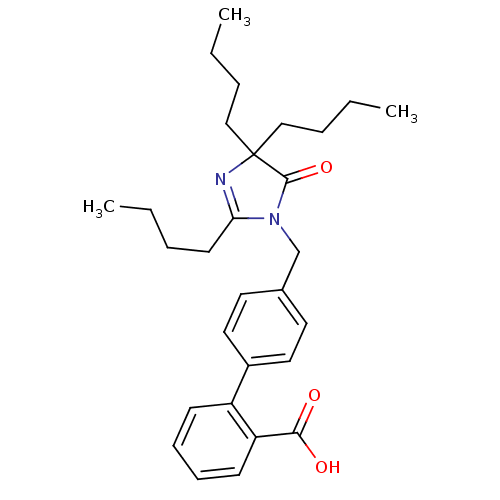
(4'-(2,4,4-Tributyl-5-oxo-4,5-dihydro-imidazol-1-yl...)Show SMILES CCCCC1=NC(CCCC)(CCCC)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H38N2O3/c1-4-7-14-26-30-29(19-8-5-2,20-9-6-3)28(34)31(26)21-22-15-17-23(18-16-22)24-12-10-11-13-25(24)27(32)33/h10-13,15-18H,4-9,14,19-21H2,1-3H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50042241
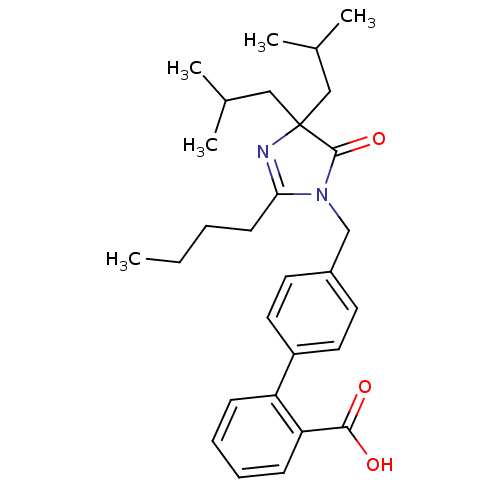
(4'-(2-Butyl-4,4-diisobutyl-5-oxo-4,5-dihydro-imida...)Show SMILES CCCCC1=NC(CC(C)C)(CC(C)C)C(=O)N1Cc1ccc(cc1)-c1ccccc1C(O)=O |t:4| Show InChI InChI=1S/C29H38N2O3/c1-6-7-12-26-30-29(17-20(2)3,18-21(4)5)28(34)31(26)19-22-13-15-23(16-14-22)24-10-8-9-11-25(24)27(32)33/h8-11,13-16,20-21H,6-7,12,17-19H2,1-5H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity at Angiotensin II receptor, type 1 from rat liver membrane by [125I]- A II displacement. |
Bioorg Med Chem Lett 4: 163-168 (1994)
Article DOI: 10.1016/S0960-894X(01)81140-9
BindingDB Entry DOI: 10.7270/Q2M045CQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data