 Found 423 hits with Last Name = 'landry' and Initial = 'dw'
Found 423 hits with Last Name = 'landry' and Initial = 'dw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241831

(CHEMBL4062273 | US10626113, Compound M | US1089975...)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H21ClN4O2/c1-14(29)28-8-7-21-18(13-28)23(17-9-15(11-25)3-5-20(17)27-21)26-12-16-4-6-22(30-2)19(24)10-16/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241840
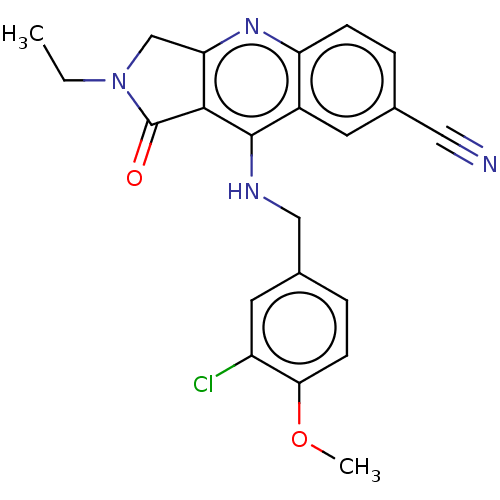
(CHEMBL4072903 | US10899756, Compound K)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N Show InChI InChI=1S/C22H19ClN4O2/c1-3-27-12-18-20(22(27)28)21(15-8-13(10-24)4-6-17(15)26-18)25-11-14-5-7-19(29-2)16(23)9-14/h4-9H,3,11-12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241840

(CHEMBL4072903 | US10899756, Compound K)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N Show InChI InChI=1S/C22H19ClN4O2/c1-3-27-12-18-20(22(27)28)21(15-8-13(10-24)4-6-17(15)26-18)25-11-14-5-7-19(29-2)16(23)9-14/h4-9H,3,11-12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM480487
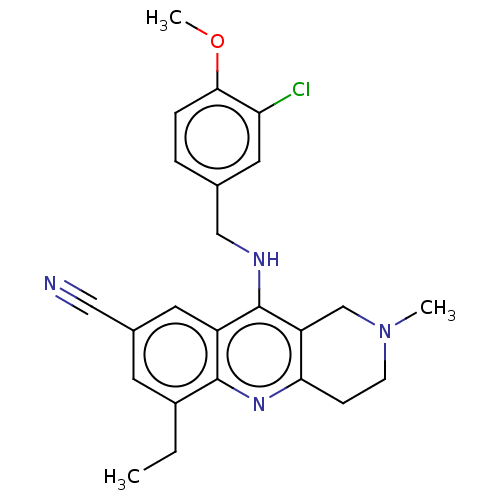
(US10626113, Compound D | US10899756, Compound D)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c3CN(C)CCc3nc12)C#N Show InChI InChI=1S/C24H25ClN4O/c1-4-17-9-16(12-26)10-18-23(17)28-21-7-8-29(2)14-19(21)24(18)27-13-15-5-6-22(30-3)20(25)11-15/h5-6,9-11H,4,7-8,13-14H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM480487

(US10626113, Compound D | US10899756, Compound D)Show SMILES CCc1cc(cc2c(NCc3ccc(OC)c(Cl)c3)c3CN(C)CCc3nc12)C#N Show InChI InChI=1S/C24H25ClN4O/c1-4-17-9-16(12-26)10-18-23(17)28-21-7-8-29(2)14-19(21)24(18)27-13-15-5-6-22(30-3)20(25)11-15/h5-6,9-11H,4,7-8,13-14H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241832
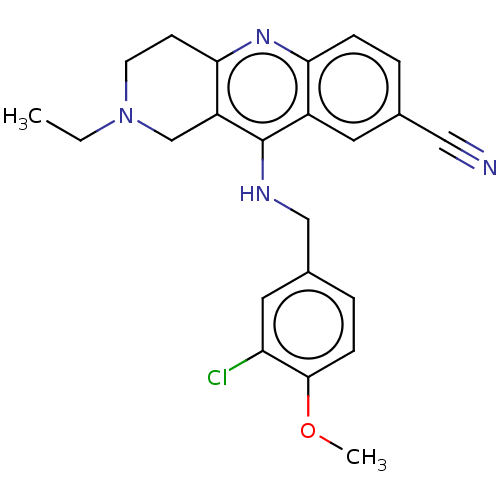
(CHEMBL4083986 | US10626113, Compound C | US1089975...)Show SMILES CCN1CCc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C23H23ClN4O/c1-3-28-9-8-21-18(14-28)23(17-10-15(12-25)4-6-20(17)27-21)26-13-16-5-7-22(29-2)19(24)11-16/h4-7,10-11H,3,8-9,13-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241832

(CHEMBL4083986 | US10626113, Compound C | US1089975...)Show SMILES CCN1CCc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C23H23ClN4O/c1-3-28-9-8-21-18(14-28)23(17-10-15(12-25)4-6-20(17)27-21)26-13-16-5-7-22(29-2)19(24)11-16/h4-7,10-11H,3,8-9,13-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241832

(CHEMBL4083986 | US10626113, Compound C | US1089975...)Show SMILES CCN1CCc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C23H23ClN4O/c1-3-28-9-8-21-18(14-28)23(17-10-15(12-25)4-6-20(17)27-21)26-13-16-5-7-22(29-2)19(24)11-16/h4-7,10-11H,3,8-9,13-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428976
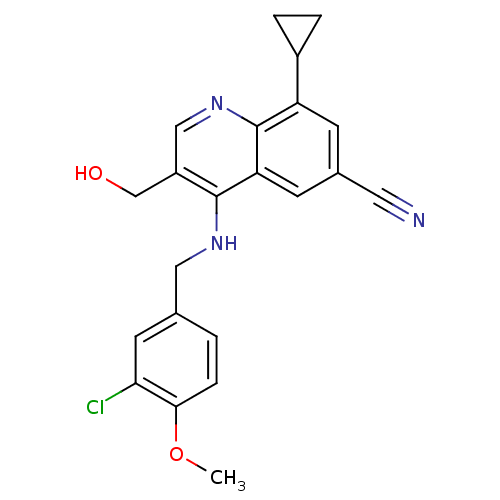
(CHEMBL2333219)Show SMILES COc1ccc(CNc2c(CO)cnc3c(cc(cc23)C#N)C2CC2)cc1Cl Show InChI InChI=1S/C22H20ClN3O2/c1-28-20-5-2-13(8-19(20)23)10-25-21-16(12-27)11-26-22-17(15-3-4-15)6-14(9-24)7-18(21)22/h2,5-8,11,15,27H,3-4,10,12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.277 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241842
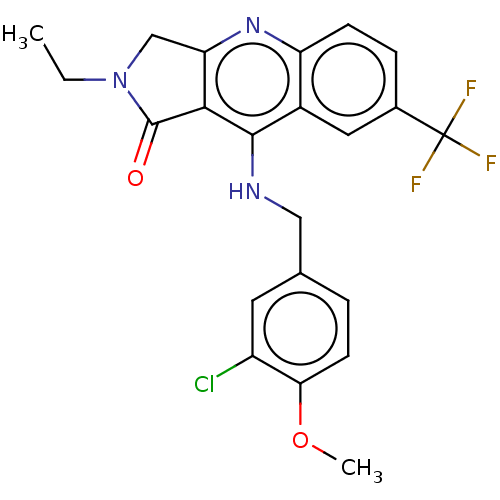
(CHEMBL4064315 | US10899756, Compound M)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C(F)(F)F Show InChI InChI=1S/C22H19ClF3N3O2/c1-3-29-11-17-19(21(29)30)20(27-10-12-4-7-18(31-2)15(23)8-12)14-9-13(22(24,25)26)5-6-16(14)28-17/h4-9H,3,10-11H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241842

(CHEMBL4064315 | US10899756, Compound M)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C(F)(F)F Show InChI InChI=1S/C22H19ClF3N3O2/c1-3-29-11-17-19(21(29)30)20(27-10-12-4-7-18(31-2)15(23)8-12)14-9-13(22(24,25)26)5-6-16(14)28-17/h4-9H,3,10-11H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of adenylate cyclase via Adenosine A1 receptor in rat fat cell membranes |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241835
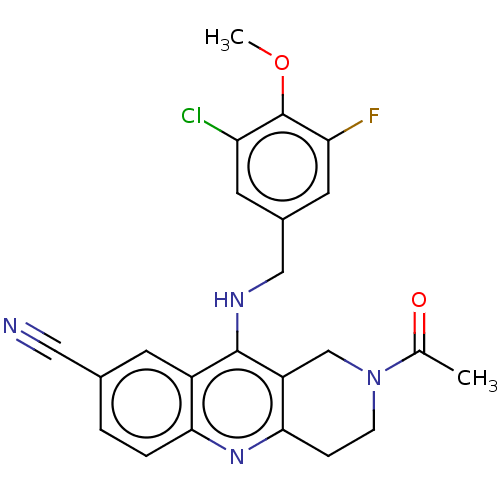
(CHEMBL4092717 | US10899756, Compound AC)Show SMILES COc1c(F)cc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H20ClFN4O2/c1-13(30)29-6-5-21-17(12-29)22(16-7-14(10-26)3-4-20(16)28-21)27-11-15-8-18(24)23(31-2)19(25)9-15/h3-4,7-9H,5-6,11-12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241835

(CHEMBL4092717 | US10899756, Compound AC)Show SMILES COc1c(F)cc(CNc2c3CN(CCc3nc3ccc(cc23)C#N)C(C)=O)cc1Cl Show InChI InChI=1S/C23H20ClFN4O2/c1-13(30)29-6-5-21-17(12-29)22(16-7-14(10-26)3-4-20(16)28-21)27-11-15-8-18(24)23(31-2)19(25)9-15/h3-4,7-9H,5-6,11-12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428975
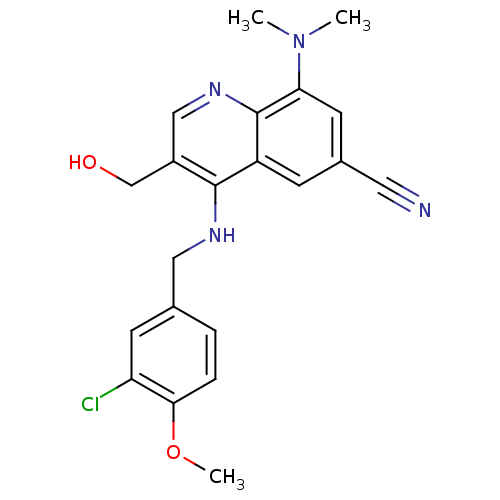
(CHEMBL2333220)Show SMILES COc1ccc(CNc2c(CO)cnc3c(cc(cc23)C#N)N(C)C)cc1Cl Show InChI InChI=1S/C21H21ClN4O2/c1-26(2)18-8-14(9-23)6-16-20(15(12-27)11-25-21(16)18)24-10-13-4-5-19(28-3)17(22)7-13/h4-8,11,27H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242157
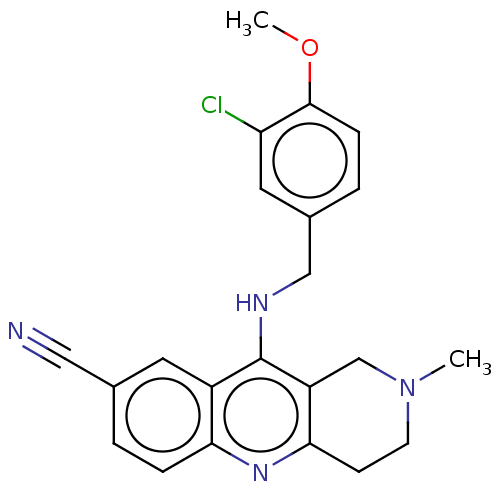
(CHEMBL4070894 | US10626113, Compound B | US1089975...)Show SMILES COc1ccc(CNc2c3CN(C)CCc3nc3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H21ClN4O/c1-27-8-7-20-17(13-27)22(16-9-14(11-24)3-5-19(16)26-20)25-12-15-4-6-21(28-2)18(23)10-15/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428971
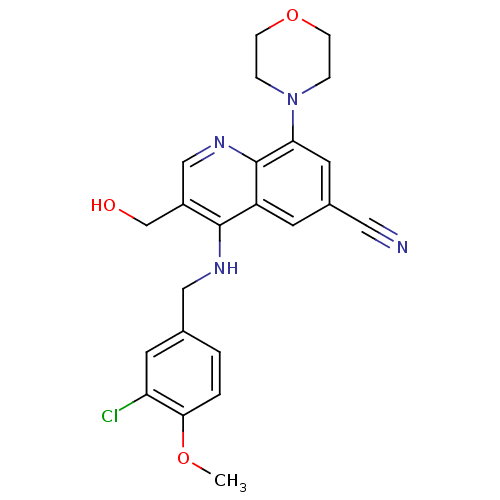
(CHEMBL2333224)Show SMILES COc1ccc(CNc2c(CO)cnc3c(cc(cc23)C#N)N2CCOCC2)cc1Cl Show InChI InChI=1S/C23H23ClN4O3/c1-30-21-3-2-15(9-19(21)24)12-26-22-17(14-29)13-27-23-18(22)8-16(11-25)10-20(23)28-4-6-31-7-5-28/h2-3,8-10,13,29H,4-7,12,14H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14776

(2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...)Show SMILES CCCc1nc(C)c2n1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(CC)CC1 Show InChI InChI=1S/C23H32N6O4S/c1-5-8-20-24-16(4)21-23(30)25-22(26-29(20)21)18-15-17(9-10-19(18)33-7-3)34(31,32)28-13-11-27(6-2)12-14-28/h9-10,15H,5-8,11-14H2,1-4H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242157

(CHEMBL4070894 | US10626113, Compound B | US1089975...)Show SMILES COc1ccc(CNc2c3CN(C)CCc3nc3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H21ClN4O/c1-27-8-7-20-17(13-27)22(16-9-14(11-24)3-5-19(16)26-20)25-12-15-4-6-21(28-2)18(23)10-15/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50242157

(CHEMBL4070894 | US10626113, Compound B | US1089975...)Show SMILES COc1ccc(CNc2c3CN(C)CCc3nc3ccc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H21ClN4O/c1-27-8-7-20-17(13-27)22(16-9-14(11-24)3-5-19(16)26-20)25-12-15-4-6-21(28-2)18(23)10-15/h3-6,9-10H,7-8,12-13H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191859

(1-adamantan-1-yl-3-[1-(2,2,2-trifluoro-acetyl)-pip...)Show SMILES FC(F)(F)C(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:15:16:19.18.23:21,THB:17:18:21:25.16.24,17:16:19.18.23:21,24:16:19:23.22.21,24:22:19:25.17.16,15:16:19:23.22.21| Show InChI InChI=1S/C18H26F3N3O2/c19-18(20,21)15(25)24-3-1-14(2-4-24)22-16(26)23-17-8-11-5-12(9-17)7-13(6-11)10-17/h11-14H,1-10H2,(H2,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241834
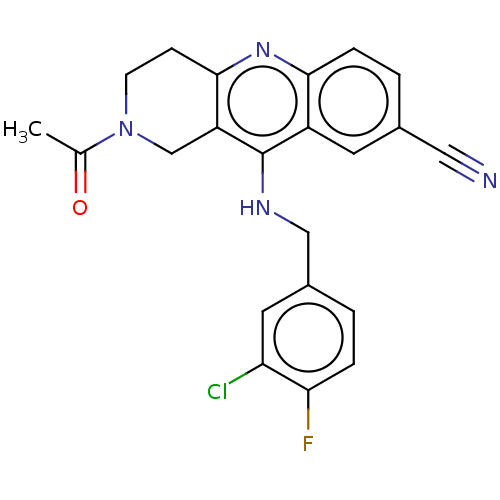
(CHEMBL4065036 | US10626113, Compound J | US1089975...)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccc(F)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C22H18ClFN4O/c1-13(29)28-7-6-21-17(12-28)22(16-8-14(10-25)3-5-20(16)27-21)26-11-15-2-4-19(24)18(23)9-15/h2-5,8-9H,6-7,11-12H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241834

(CHEMBL4065036 | US10626113, Compound J | US1089975...)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccc(F)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C22H18ClFN4O/c1-13(29)28-7-6-21-17(12-28)22(16-8-14(10-25)3-5-20(16)27-21)26-11-15-2-4-19(24)18(23)9-15/h2-5,8-9H,6-7,11-12H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241834

(CHEMBL4065036 | US10626113, Compound J | US1089975...)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3ccc(F)c(Cl)c3)c2C1)C#N Show InChI InChI=1S/C22H18ClFN4O/c1-13(29)28-7-6-21-17(12-28)22(16-8-14(10-25)3-5-20(16)27-21)26-11-15-2-4-19(24)18(23)9-15/h2-5,8-9H,6-7,11-12H2,1H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241837

(CHEMBL4102913 | US10626113, Compound G | US1089975...)Show InChI InChI=1S/C21H19ClN4O/c1-27-20-5-3-14(9-17(20)22)11-25-21-15-8-13(10-23)2-4-18(15)26-19-6-7-24-12-16(19)21/h2-5,8-9,24H,6-7,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241837

(CHEMBL4102913 | US10626113, Compound G | US1089975...)Show InChI InChI=1S/C21H19ClN4O/c1-27-20-5-3-14(9-17(20)22)11-25-21-15-8-13(10-23)2-4-18(15)26-19-6-7-24-12-16(19)21/h2-5,8-9,24H,6-7,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25732

(3-adamantan-1-yl-1-cyclohexylurea | CHEMBL242255 |...)Show SMILES O=C(NC1CCCCC1)NC12CC3CC(CC(C3)C1)C2 |TLB:17:12:19:16.15.18,17:16:12.13.11:19,THB:15:14:11:16.17.18,15:16:11:14.13.19| Show InChI InChI=1S/C17H28N2O/c20-16(18-15-4-2-1-3-5-15)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,1-11H2,(H2,18,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241837

(CHEMBL4102913 | US10626113, Compound G | US1089975...)Show InChI InChI=1S/C21H19ClN4O/c1-27-20-5-3-14(9-17(20)22)11-25-21-15-8-13(10-23)2-4-18(15)26-19-6-7-24-12-16(19)21/h2-5,8-9,24H,6-7,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50360348
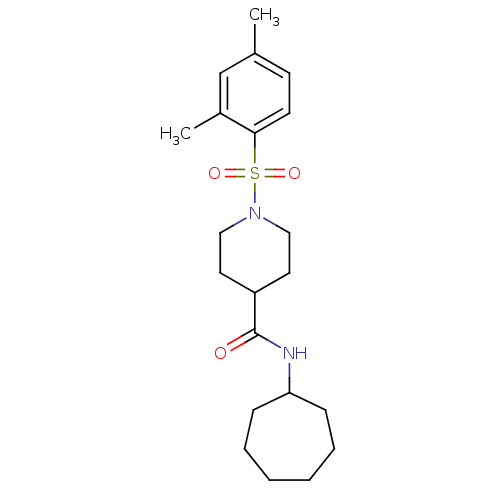
(CHEMBL1933651)Show SMILES Cc1ccc(c(C)c1)S(=O)(=O)N1CCC(CC1)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C21H32N2O3S/c1-16-9-10-20(17(2)15-16)27(25,26)23-13-11-18(12-14-23)21(24)22-19-7-5-3-4-6-8-19/h9-10,15,18-19H,3-8,11-14H2,1-2H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241843
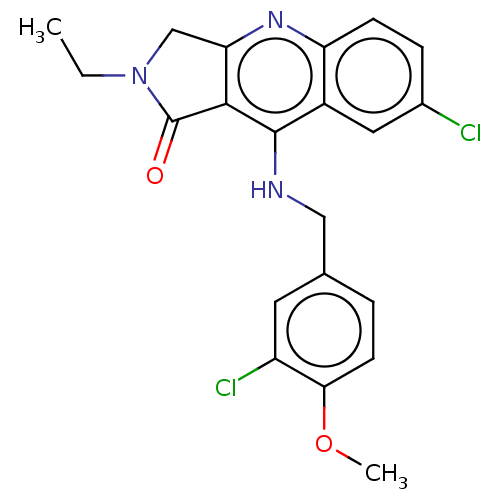
(CHEMBL4102250 | US10899756, Compound N)Show SMILES CCN1Cc2nc3ccc(Cl)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C21H19Cl2N3O2/c1-3-26-11-17-19(21(26)27)20(14-9-13(22)5-6-16(14)25-17)24-10-12-4-7-18(28-2)15(23)8-12/h4-9H,3,10-11H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241843

(CHEMBL4102250 | US10899756, Compound N)Show SMILES CCN1Cc2nc3ccc(Cl)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C21H19Cl2N3O2/c1-3-26-11-17-19(21(26)27)20(14-9-13(22)5-6-16(14)25-17)24-10-12-4-7-18(28-2)15(23)8-12/h4-9H,3,10-11H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737
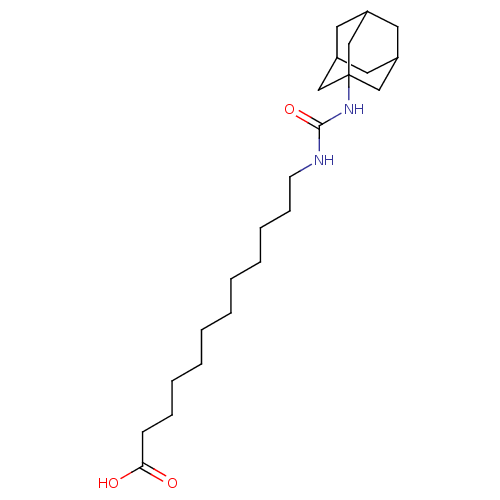
(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241839
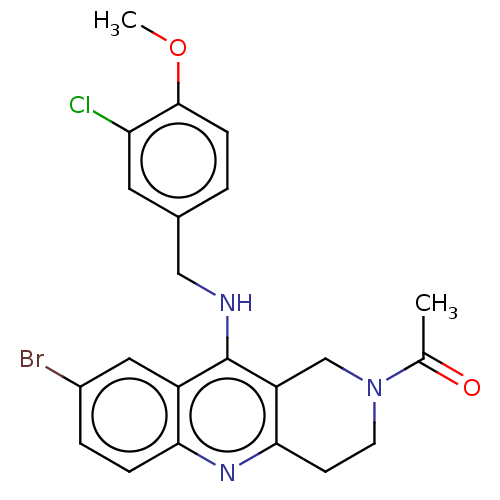
(CHEMBL4083346)Show SMILES COc1ccc(CNc2c3CN(CCc3nc3ccc(Br)cc23)C(C)=O)cc1Cl Show InChI InChI=1S/C22H21BrClN3O2/c1-13(28)27-8-7-20-17(12-27)22(16-10-15(23)4-5-19(16)26-20)25-11-14-3-6-21(29-2)18(24)9-14/h3-6,9-10H,7-8,11-12H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM480506

(US10899756, Compound AB)Show SMILES CC(=O)N1CCc2nc3ccc(cc3c(NCc3cc(F)c(F)c(F)c3)c2C1)C#N Show InChI InChI=1S/C22H17F3N4O/c1-12(30)29-5-4-20-16(11-29)22(15-6-13(9-26)2-3-19(15)28-20)27-10-14-7-17(23)21(25)18(24)8-14/h2-3,6-8H,4-5,10-11H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241841

(CHEMBL4092040 | US10899756, Compound L)Show SMILES CCN1Cc2nc3ccc(OC)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C22H22ClN3O3/c1-4-26-12-18-20(22(26)27)21(15-10-14(28-2)6-7-17(15)25-18)24-11-13-5-8-19(29-3)16(23)9-13/h5-10H,4,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50241841

(CHEMBL4092040 | US10899756, Compound L)Show SMILES CCN1Cc2nc3ccc(OC)cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O Show InChI InChI=1S/C22H22ClN3O3/c1-4-26-12-18-20(22(26)27)21(15-10-14(28-2)6-7-17(15)25-18)24-11-13-5-8-19(29-3)16(23)9-13/h5-10H,4,11-12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE5A1 using FAM-labelled cGMP as substrate after 60 mins by fluorescence polarization assay |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428972

(CHEMBL2333223)Show SMILES COc1ccc(CNc2c(CO)cnc3c(NC4CC4)cc(cc23)C#N)cc1Cl Show InChI InChI=1S/C22H21ClN4O2/c1-29-20-5-2-13(7-18(20)23)10-25-21-15(12-28)11-26-22-17(21)6-14(9-24)8-19(22)27-16-3-4-16/h2,5-8,11,16,27-28H,3-4,10,12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50428974
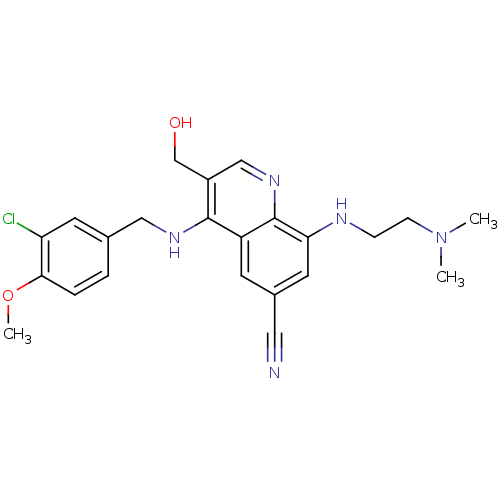
(CHEMBL2333221)Show SMILES COc1ccc(CNc2c(CO)cnc3c(NCCN(C)C)cc(cc23)C#N)cc1Cl Show InChI InChI=1S/C23H26ClN5O2/c1-29(2)7-6-26-20-10-16(11-25)8-18-22(17(14-30)13-28-23(18)20)27-12-15-4-5-21(31-3)19(24)9-15/h4-5,8-10,13,26,30H,6-7,12,14H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of PDE5 (unknown origin) using FAM-cGMP as substrate after 60 mins by fluorescence assay |
Eur J Med Chem 60: 285-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.009
BindingDB Entry DOI: 10.7270/Q22F7PSZ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191878

(1-(1-Acetyl-piperidin-4-ylmethyl)-3-adamantan-1-yl...)Show SMILES CC(=O)N1CCC(CNC(=O)NC23CC4CC(CC(C4)C2)C3)CC1 |TLB:11:12:15.14.19:17,THB:13:14:17:21.12.20,13:12:15.14.19:17,20:12:15:19.18.17,20:18:15:21.13.12| Show InChI InChI=1S/C19H31N3O2/c1-13(23)22-4-2-14(3-5-22)12-20-18(24)21-19-9-15-6-16(10-19)8-17(7-15)11-19/h14-17H,2-12H2,1H3,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of epoxide hydrolase (unknown origin) |
Bioorg Med Chem Lett 19: 2354-9 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.066
BindingDB Entry DOI: 10.7270/Q2J10322 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM480489

(US10626113, Compound F | US10899756, Compound F)Show InChI InChI=1S/C20H19BrClN3O/c1-26-19-5-2-12(8-16(19)22)10-24-20-14-9-13(21)3-4-17(14)25-18-6-7-23-11-15(18)20/h2-5,8-9,23H,6-7,10-11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of Columbia University in the City of New York
US Patent
| Assay Description
A series of dilutions of the test compounds were prepared with 10% DMSO in assay buffer and 5 μl of the dilution was added to a 50 μl react... |
US Patent US10899756 (2021)
BindingDB Entry DOI: 10.7270/Q2RN3C0P |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM480489

(US10626113, Compound F | US10899756, Compound F)Show InChI InChI=1S/C20H19BrClN3O/c1-26-19-5-2-12(8-16(19)22)10-24-20-14-9-13(21)3-4-17(14)25-18-6-7-23-11-15(18)20/h2-5,8-9,23H,6-7,10-11H2,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2KH0SFD |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50360341
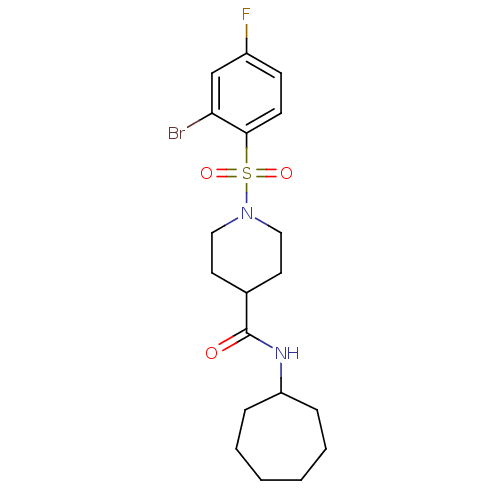
(CHEMBL1933515)Show SMILES Fc1ccc(c(Br)c1)S(=O)(=O)N1CCC(CC1)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C19H26BrFN2O3S/c20-17-13-15(21)7-8-18(17)27(25,26)23-11-9-14(10-12-23)19(24)22-16-5-3-1-2-4-6-16/h7-8,13-14,16H,1-6,9-12H2,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
Cone cGMP-specific 3',5'-cyclic phosphodiesterase subunit alpha'
(Homo sapiens (Human)) | BDBM50241840

(CHEMBL4072903 | US10899756, Compound K)Show SMILES CCN1Cc2nc3ccc(cc3c(NCc3ccc(OC)c(Cl)c3)c2C1=O)C#N Show InChI InChI=1S/C22H19ClN4O2/c1-3-27-12-18-20(22(27)28)21(15-8-13(10-24)4-6-17(15)26-18)25-11-14-5-7-19(29-2)16(23)9-14/h4-9H,3,11-12H2,1-2H3,(H,25,26) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the activity of K+ stimulated gastric ATPase |
J Med Chem 60: 8858-8875 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00979
BindingDB Entry DOI: 10.7270/Q2J67K3P |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50360321
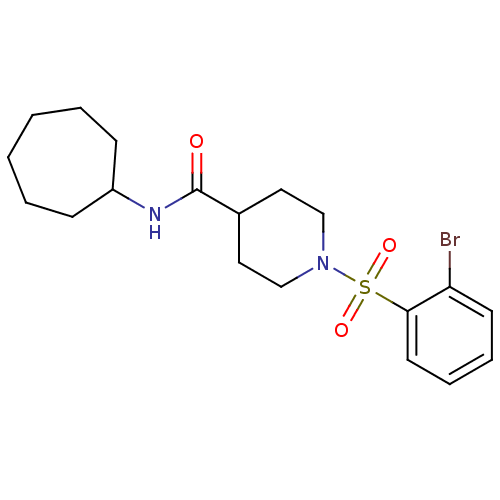
(CHEMBL1933497)Show SMILES Brc1ccccc1S(=O)(=O)N1CCC(CC1)C(=O)NC1CCCCCC1 Show InChI InChI=1S/C19H27BrN2O3S/c20-17-9-5-6-10-18(17)26(24,25)22-13-11-15(12-14-22)19(23)21-16-7-3-1-2-4-8-16/h5-6,9-10,15-16H,1-4,7-8,11-14H2,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50191854
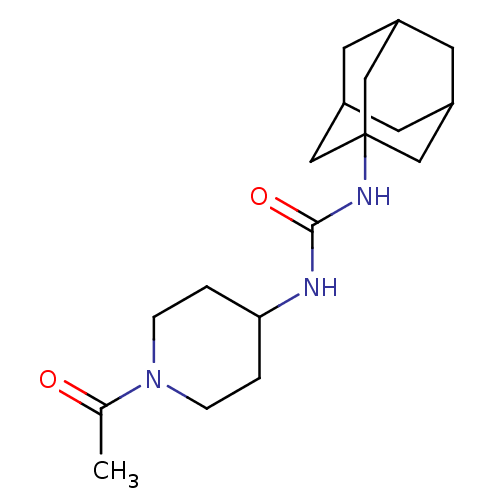
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Columbia University
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase using CMNPC as substrate after 5 mins by fluorescent assay |
Bioorg Med Chem Lett 22: 601-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.074
BindingDB Entry DOI: 10.7270/Q2TM7BJ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data