

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50398716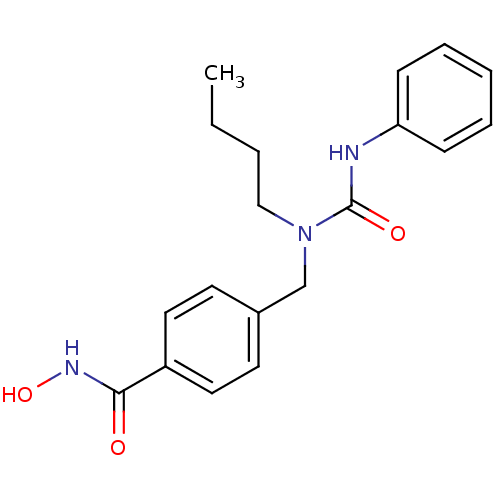 (CHEMBL2179618 | US10227295, Compound 5g | US940985...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC6 expressed in HEK293T/17 using Ac-Gly-Ala-[Ac-Lys]-AMC as substrate preincubated for 10 mins followe... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50503977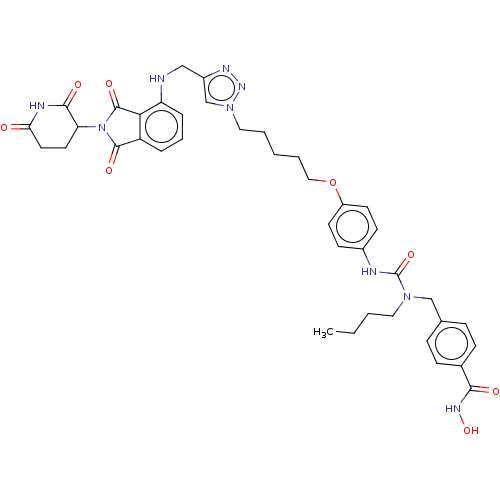 (CHEMBL4465902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC6 expressed in HEK293T/17 using Ac-Gly-Ala-[Ac-Lys]-AMC as substrate preincubated for 10 mins followe... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 7 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC7 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by s... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC9 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by s... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 11 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC11 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by ... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC8 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by s... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 5 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC5 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by s... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC1 expressed in HEK293T/17 using Ac-Gly-Ala-[Ac-Lys]-AMC as substrate preincubated for 10 mins followe... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50503977 (CHEMBL4465902) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Biotechnology of the Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human N-terminal tagged human HDAC4 expressed in HEK293T/17 using Boc-[TFA-Lys]-AMC as substrate preincubated for 10 mins followed by s... | J Med Chem 62: 7042-7057 (2019) Article DOI: 10.1021/acs.jmedchem.9b00516 BindingDB Entry DOI: 10.7270/Q2MP56H6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50541261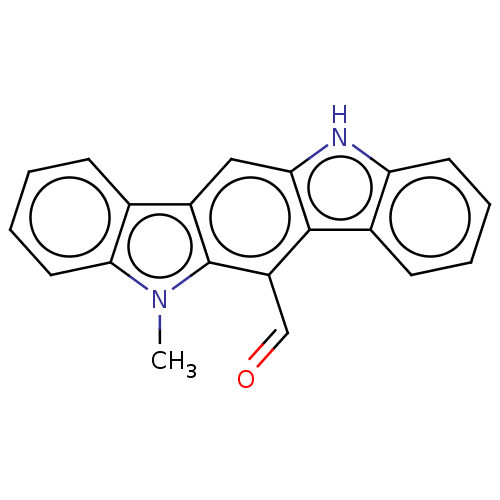 (CHEMBL4646273) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126959 BindingDB Entry DOI: 10.7270/Q2WQ07B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Homo sapiens (Human)) | BDBM50541262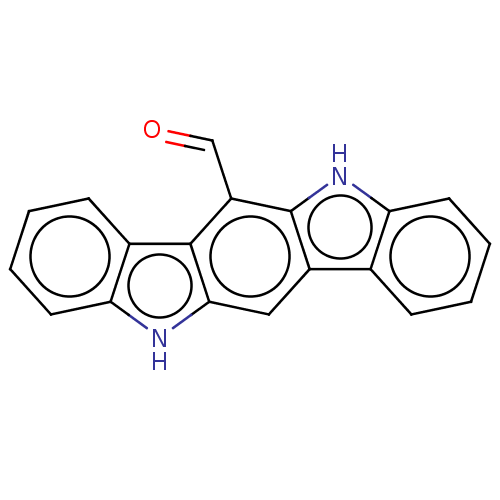 (6-Formylindolo[3,2-B]Carbazole | CHEMBL472031) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Agonist activity at AhR in human HepG2 cells assessed as induction of CYP1A1 expression after 24 hrs by ethoxyresorufin-O-deethylase assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126959 BindingDB Entry DOI: 10.7270/Q2WQ07B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||