

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50367250 (3-DEAZAARISTEROMYCIN A | CHEMBL268272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50088426 ((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182452 (CHEMBL3818567) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182448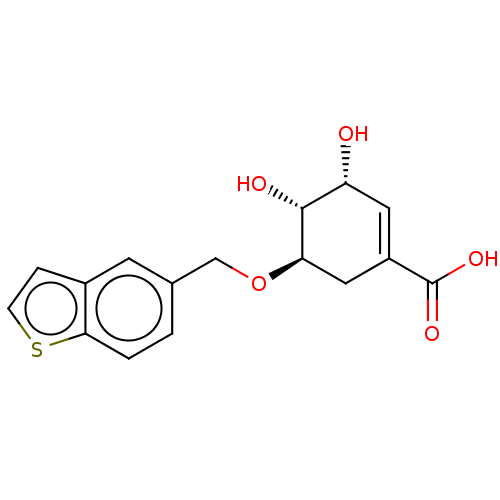 (CHEMBL3818824) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182479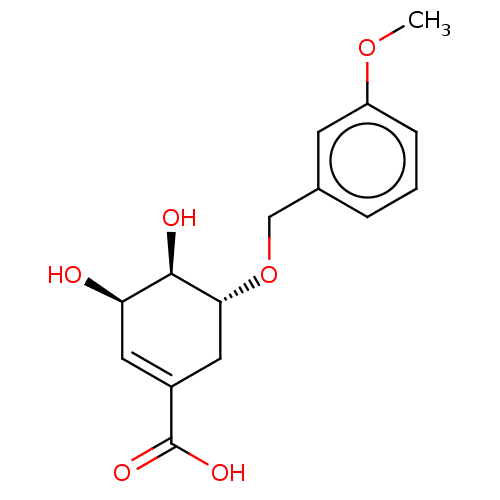 (CHEMBL3818668) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182478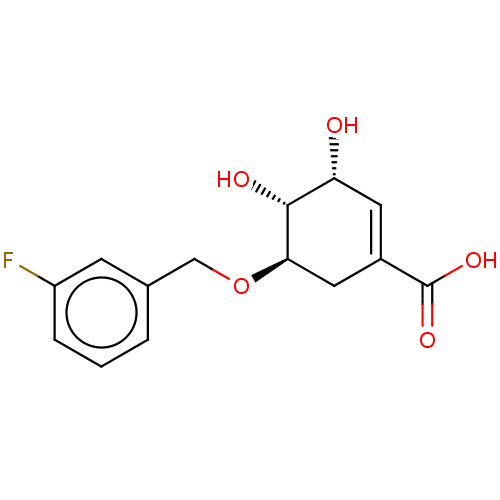 (CHEMBL3819175) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182449 (CHEMBL3819573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182476 (CHEMBL3818740) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182480 (CHEMBL3818794) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137070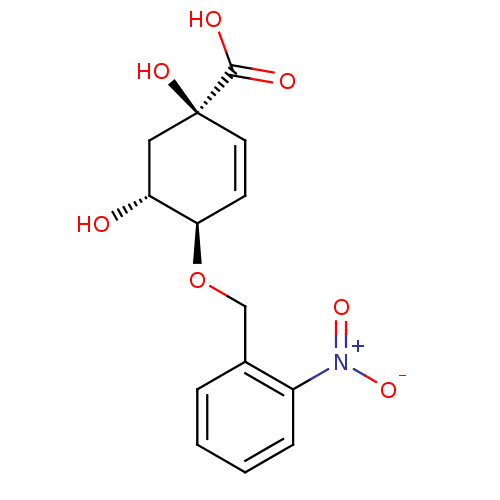 ((1R,4R,5R)-1,5-Dihydroxy-4-(2-nitro-benzyloxy)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182451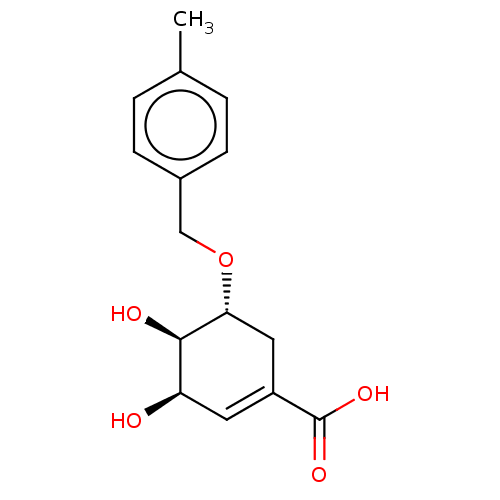 (CHEMBL3818120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50026329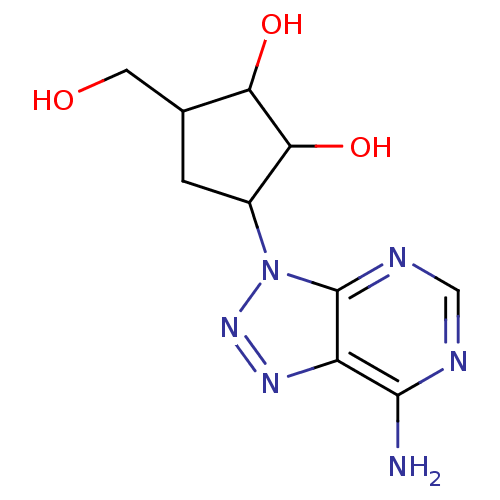 (3-(7-Amino-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182450 (CHEMBL3818962) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137066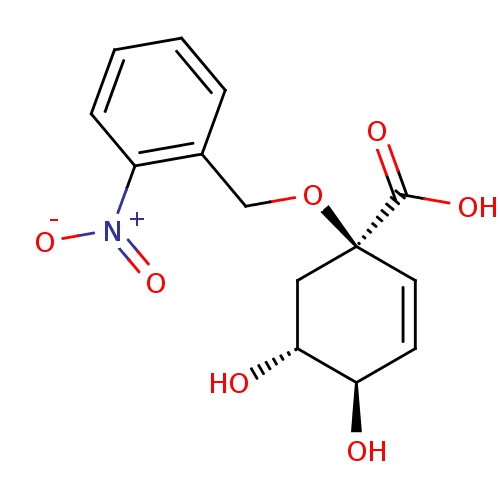 ((1R,4R,5R)-4,5-Dihydroxy-1-(2-nitro-benzyloxy)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182481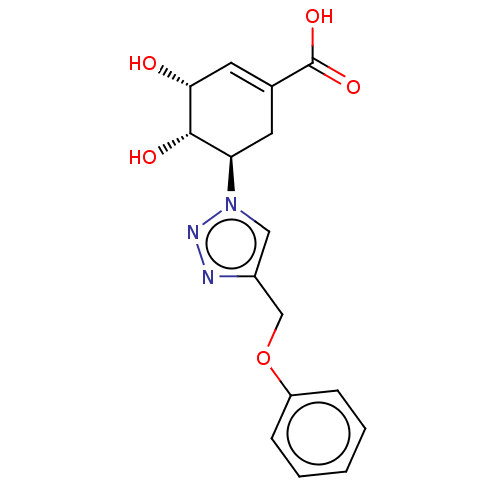 (CHEMBL3818698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137053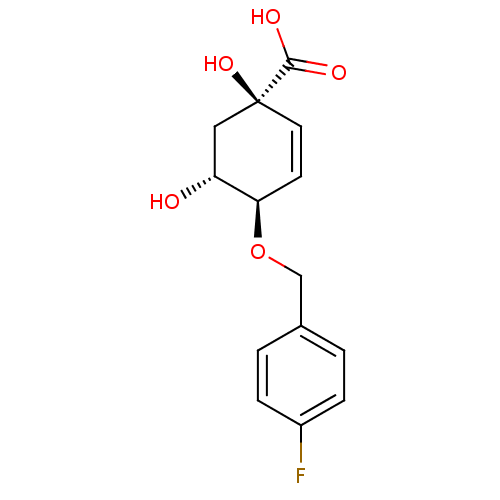 ((1R,4R,5R)-4-(4-Fluoro-benzyloxy)-1,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182447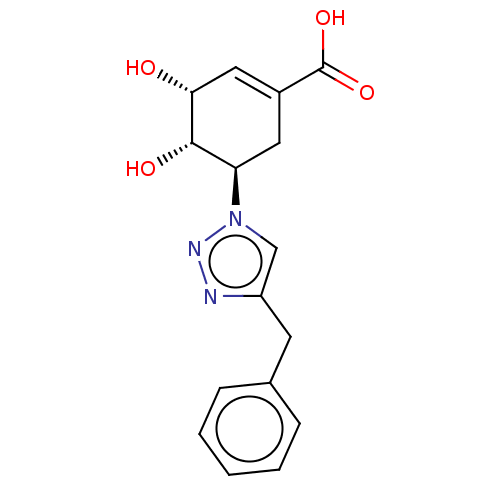 (CHEMBL3819604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137056 (4-((1R,4R,6R)-4-Carboxy-4,6-dihydroxy-cyclohex-2-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137068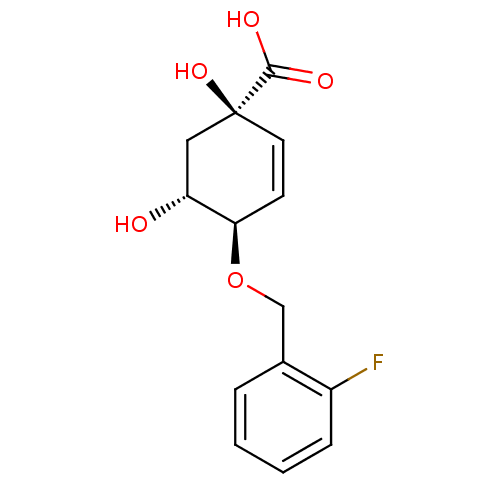 ((1R,4R,5R)-4-(2-Fluoro-benzyloxy)-1,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137055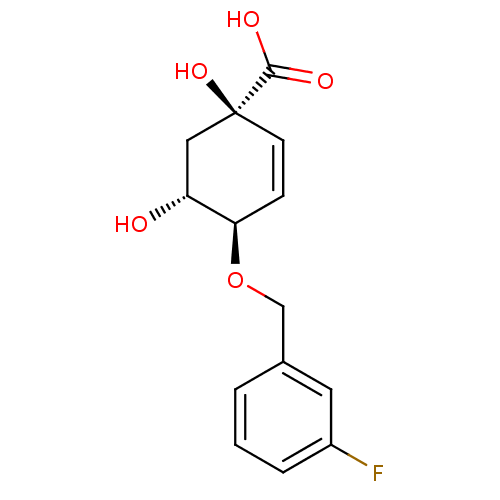 ((1R,4R,5R)-4-(3-Fluoro-benzyloxy)-1,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137059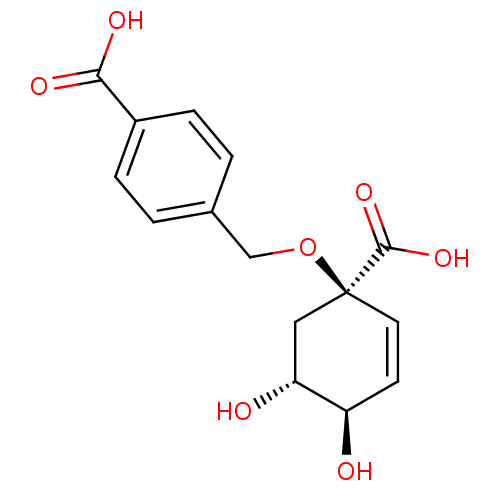 (4-((1R,4R,5R)-1-Carboxy-4,5-dihydroxy-cyclohex-2-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137054 ((1R,4R,5R)-1,5-Dihydroxy-4-(4-nitro-benzyloxy)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137067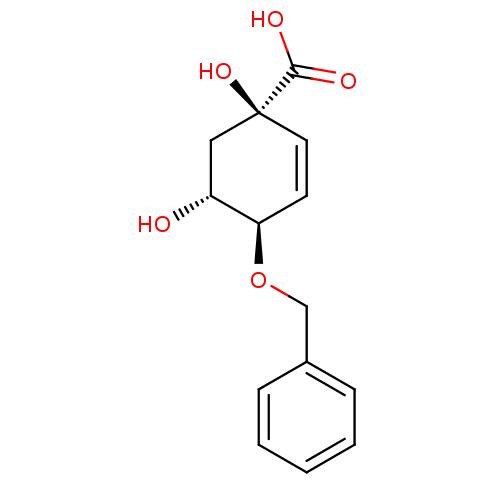 ((1R,4R,5R)-4-Benzyloxy-1,5-dihydroxy-cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137062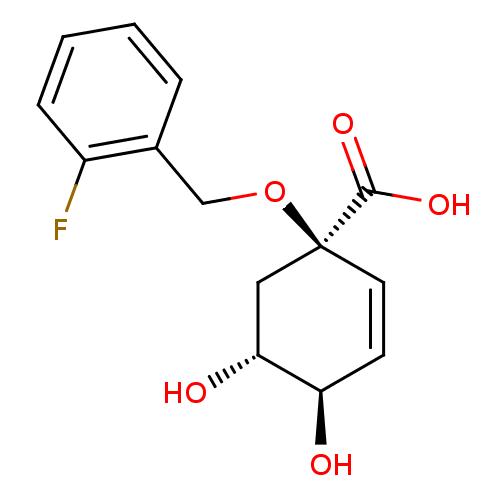 ((1R,4R,5R)-1-(2-Fluoro-benzyloxy)-4,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137060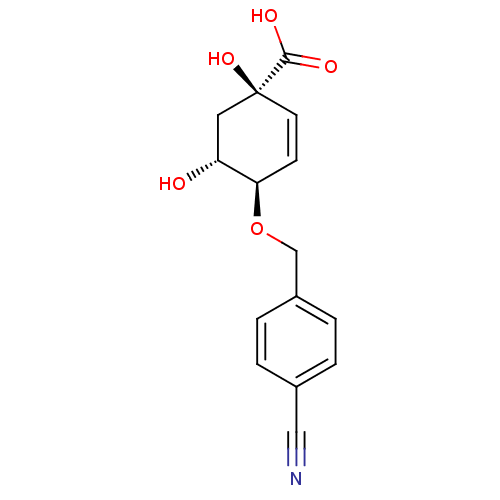 ((1R,4R,5R)-4-(4-Cyano-benzyloxy)-1,5-dihydroxy-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137063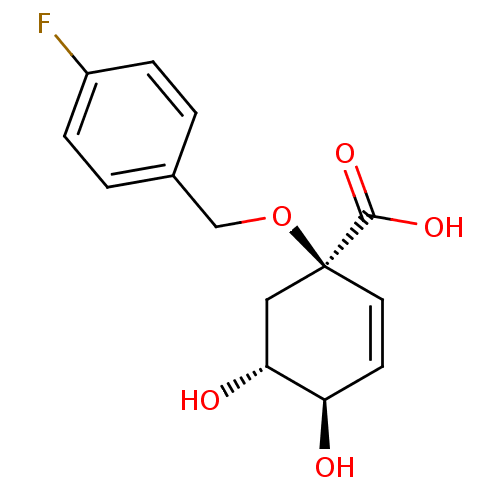 ((1R,4R,5R)-1-(4-Fluoro-benzyloxy)-4,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137057 ((1R,4R,5R)-1-Benzyloxy-4,5-dihydroxy-cyclohex-2-en...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137065 ((1R,4R,5R)-1-(3-Fluoro-benzyloxy)-4,5-dihydroxy-cy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137069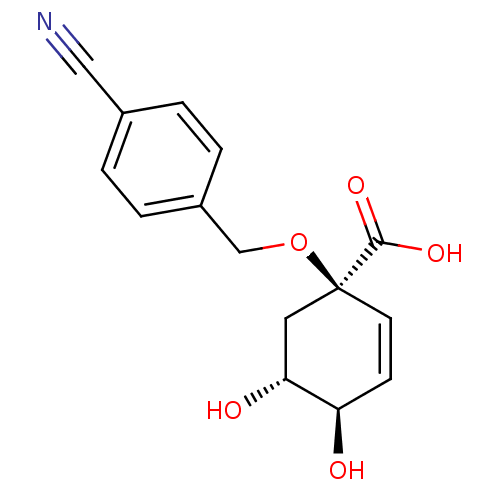 ((1R,4R,5R)-1-(4-Cyano-benzyloxy)-4,5-dihydroxy-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182483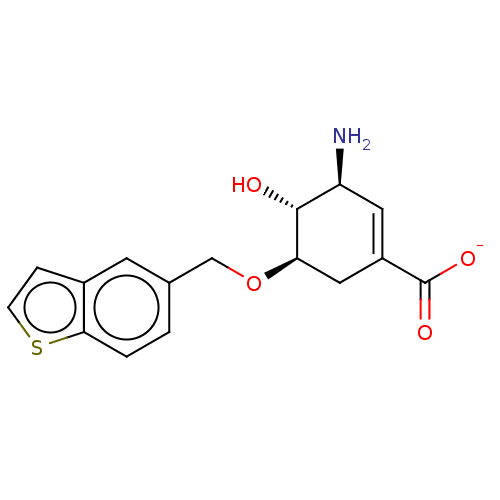 (CHEMBL3819040) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Shikimate kinase (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50182482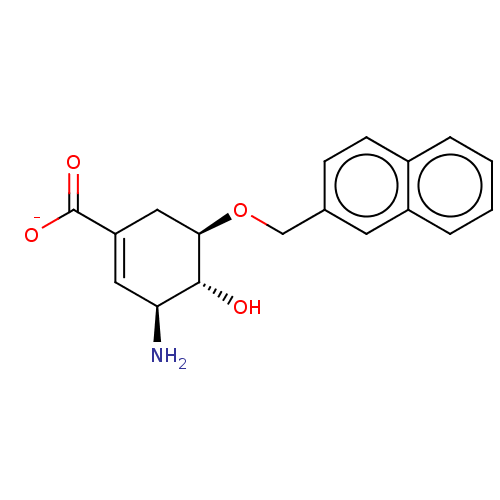 (CHEMBL3817864) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Santiago de Compostela Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori shikimate kinase using shikimic acid substrate by measuring oxidation of NADH to NAD by pyruvate kinase and lactate... | J Med Chem 59: 5471-87 (2016) Article DOI: 10.1021/acs.jmedchem.6b00483 BindingDB Entry DOI: 10.7270/Q2JW8GT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137061 ((1R,4R,5R)-4,5-Dihydroxy-1-(4-nitro-benzyloxy)-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137064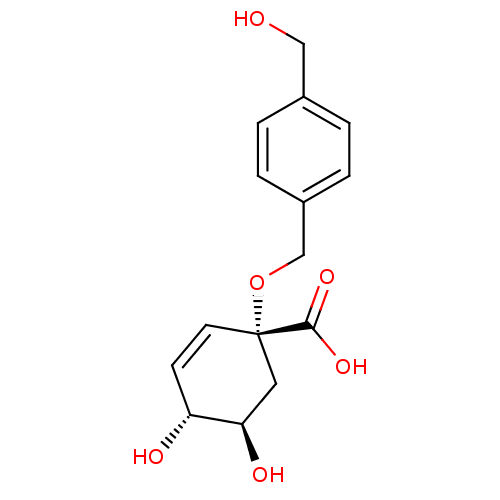 ((1R,4R,5R)-4,5-Dihydroxy-1-(4-hydroxymethyl-benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.33E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-dehydroquinate dehydratase (Streptomyces coelicolor) | BDBM50137058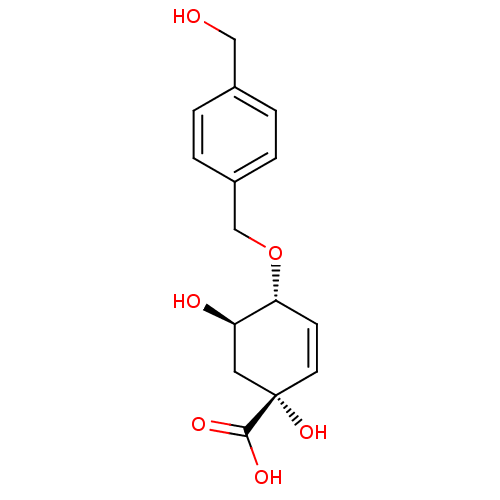 ((1R,4R,5R)-1,5-Dihydroxy-4-(4-hydroxymethyl-benzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela Curated by ChEMBL | Assay Description In vitro inhibitory activity against Streptomyces coelicolor type II dehydroquinase. | J Med Chem 46: 5735-44 (2003) Article DOI: 10.1021/jm030987q BindingDB Entry DOI: 10.7270/Q2ZS2VW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020299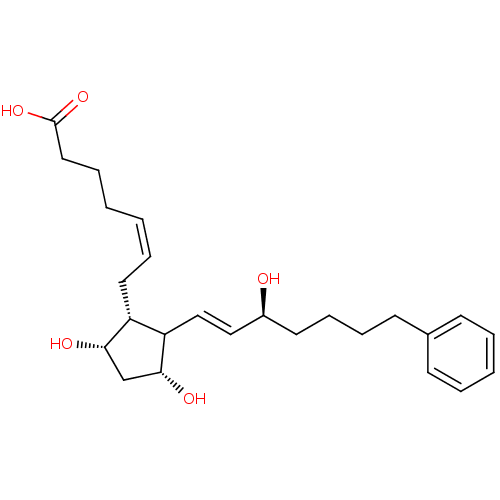 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50035622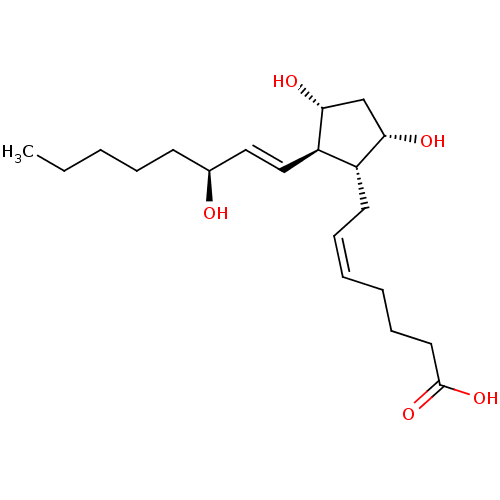 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50405735 (CHEMBL2114230) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50035622 ((5Z,13E,15S)-9alpha,11alpha,15-trihydroxyprosta-5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes... | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020299 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020302 ((S-isomer)7-{2-[5-(4-Azido-phenyl)-3-hydroxy-pent-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020303 ((R-isomer)7-{2-[5-(4-Azido-3-iodo-phenyl)-3-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50405735 (CHEMBL2114230) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (BOVINE) | BDBM50020301 ((R-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-7-phenyl-h...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards PGF-2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in bovine corpora lutea plasma membranes (BCLM) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603972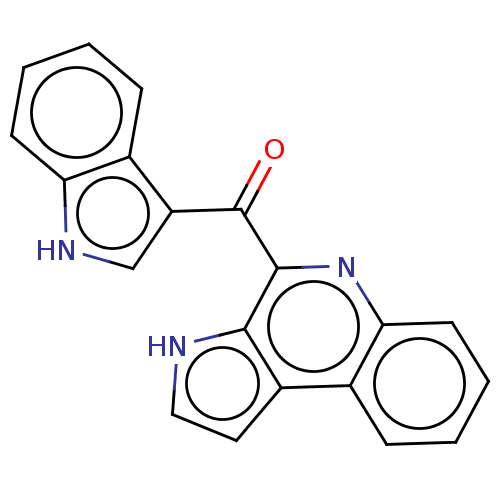 (CHEBI:67844 | MARINOQUINOLINE F | Marinoquinoline ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603971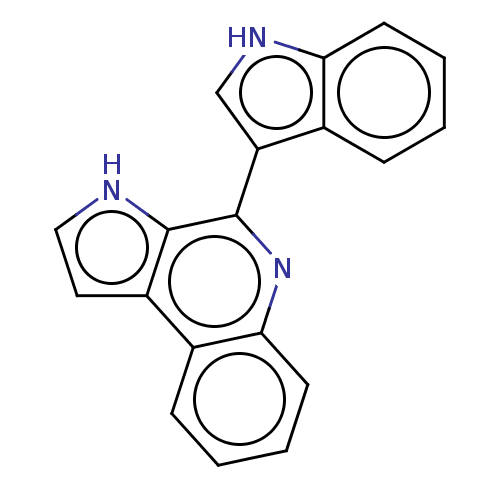 (CHEBI:67843 | MARINOQUINOLINE E | Marinoquinoline ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603970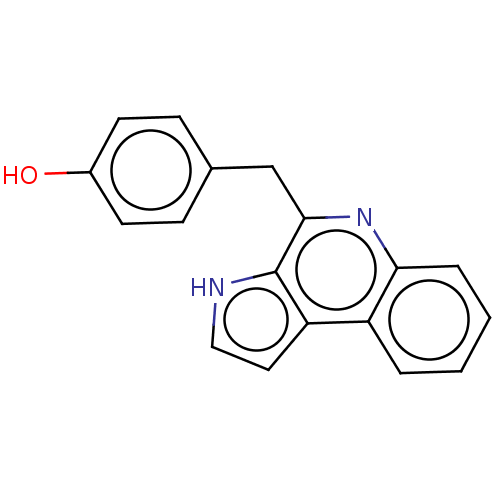 (CHEBI:67842 | MARINOQUINOLINE D | Marinoquinoline ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603969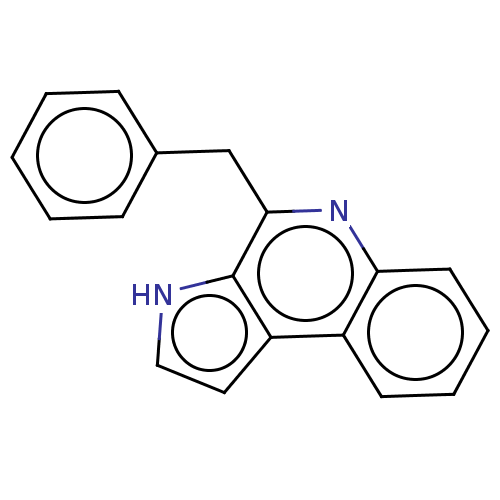 (CHEBI:67841 | MARINOQUINOLINE C | Marinoquinoline ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603968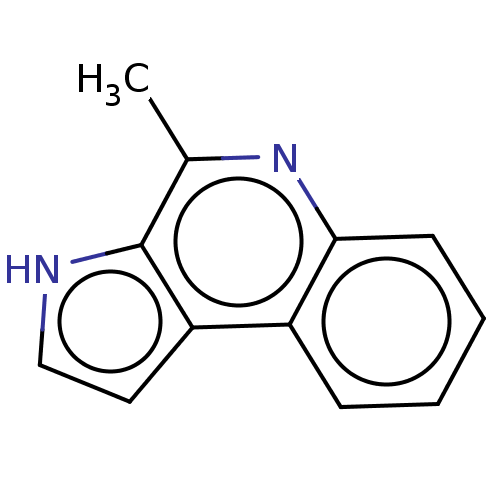 (CHEBI:67839 | MARINOQUINOLINE A | Marinoquinoline ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50603967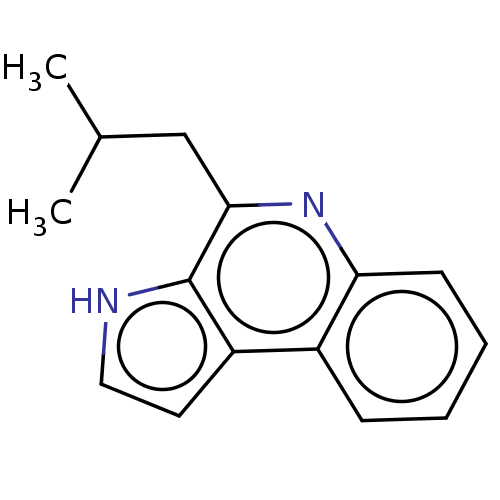 (CHEMBL4169239) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114206 BindingDB Entry DOI: 10.7270/Q2RR23BH | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50020306 ((S-isomer)7-{2-[5-(4-Azido-2-hydroxy-phenyl)-3-hyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky Curated by ChEMBL | Assay Description Binding potency towards Prostaglandin F2 alpha receptor (competitive binding) with natural [3H]-PGF 2 alpha in ovine luteal cells (OLC) | J Med Chem 32: 256-64 (1989) BindingDB Entry DOI: 10.7270/Q23F4Q7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |