

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM50546794 (CHEMBL4777335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546794 (CHEMBL4777335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176555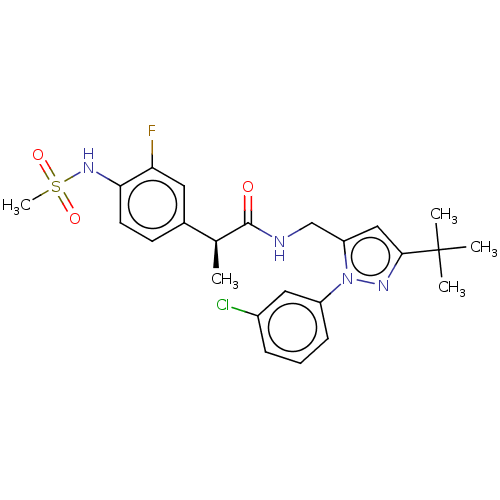 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342149 (1-((2-(tert-butyl)-4-(3-chlorophenyl)thiazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564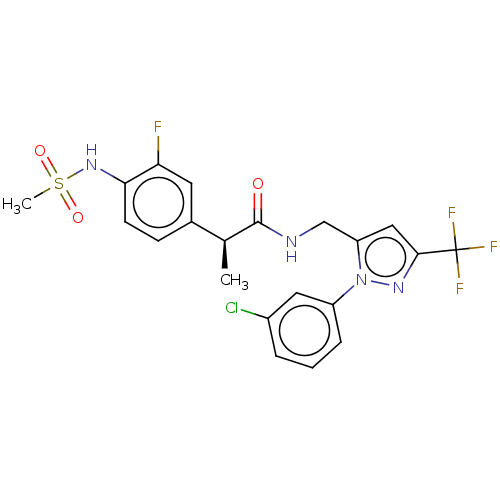 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176554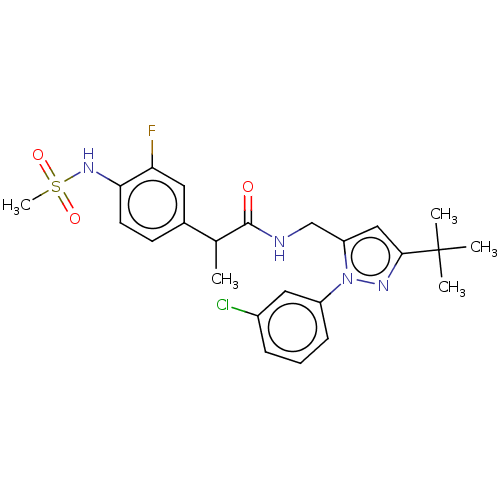 (US9120756, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546795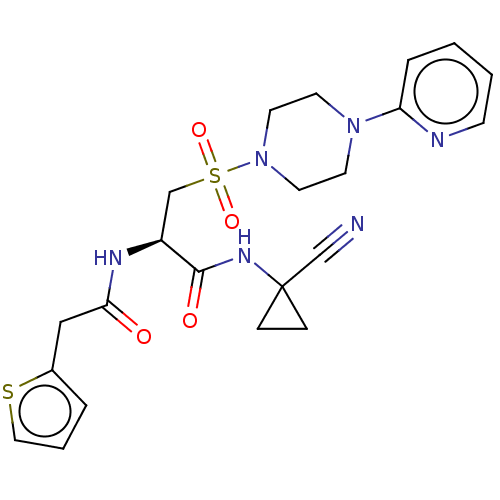 (CHEMBL4777740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546795 (CHEMBL4777740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546796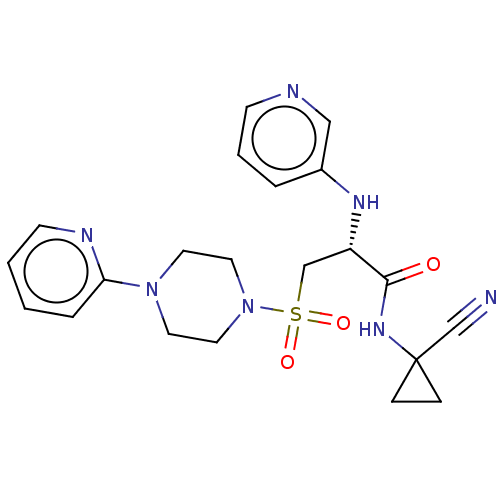 (CHEMBL4761229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50546796 (CHEMBL4761229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human Cathepsin S expressed in baculovirus infected insect cells using Z-VVR-AMC as substrate preincubated for 15 mins foll... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00949 BindingDB Entry DOI: 10.7270/Q2NV9NVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176554 (US9120756, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176561 (US9120756, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176562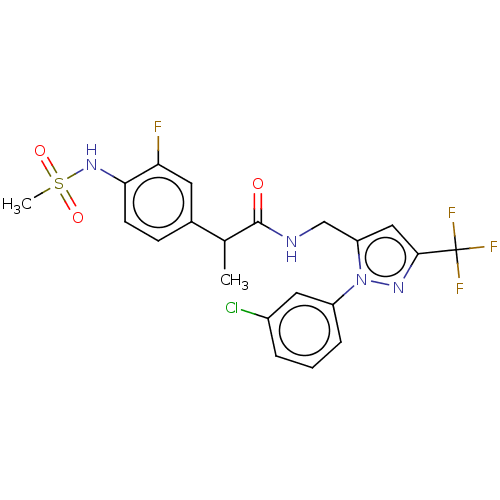 (US9120756, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176559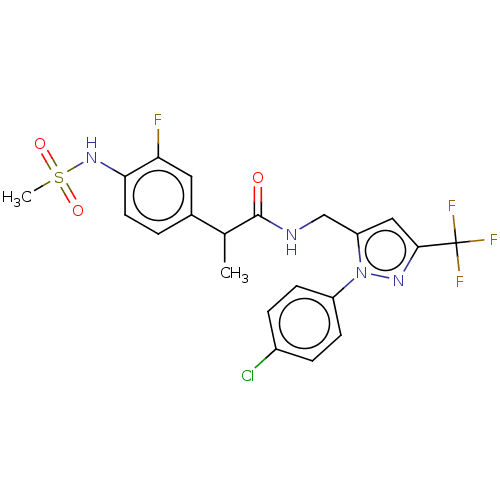 (US9120756, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553855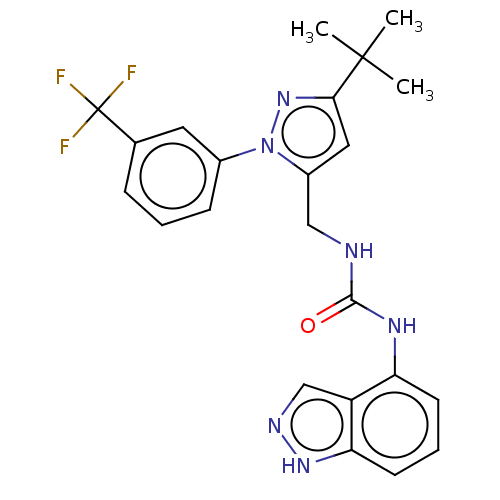 (CHEMBL4782906) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50232114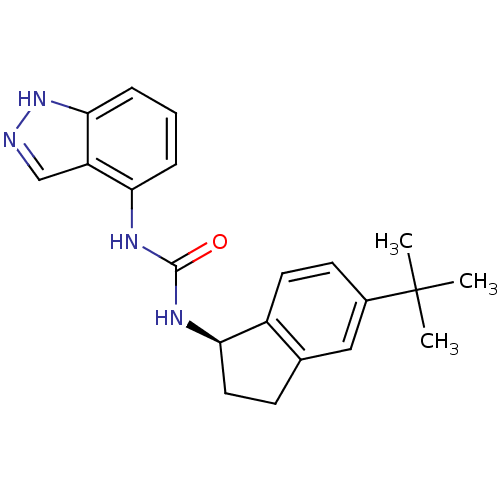 ((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50398494 (CHEMBL2177429) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced TRPV1 activation by FLIPR method | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128266 BindingDB Entry DOI: 10.7270/Q2CV4NG9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342128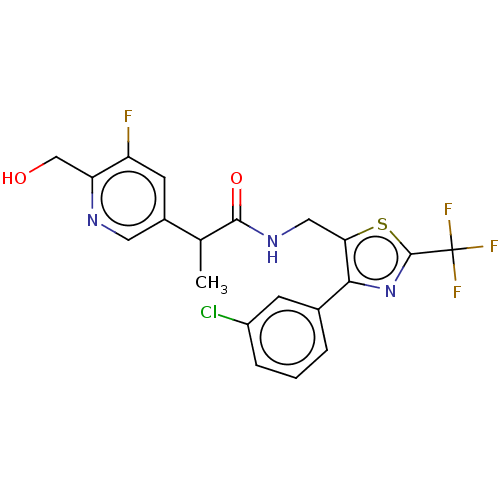 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176549 (US9120756, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176562 (US9120756, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176566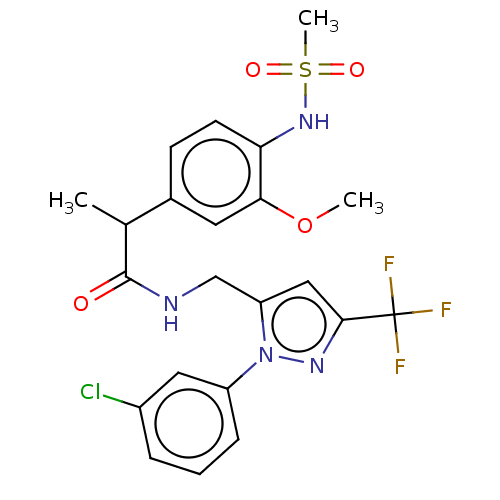 (US9120756, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342122 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553837 (CHEMBL4752231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176561 (US9120756, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176556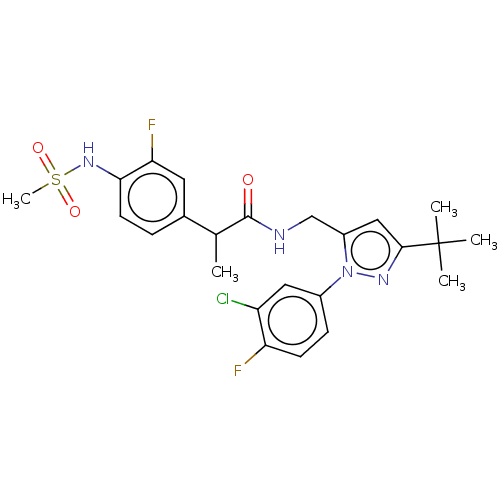 (US9120756, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553860 (CHEMBL4745407) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553861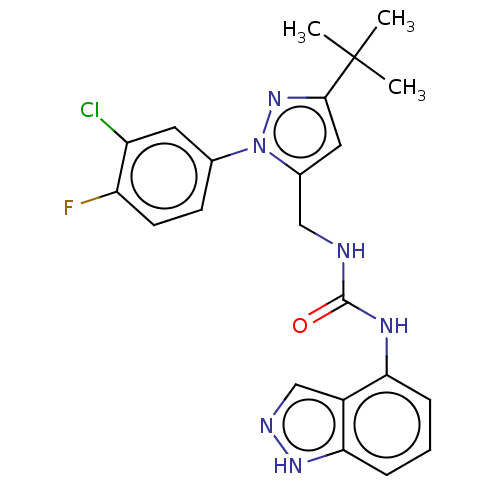 (CHEMBL4792344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553868 (CHEMBL4763969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553862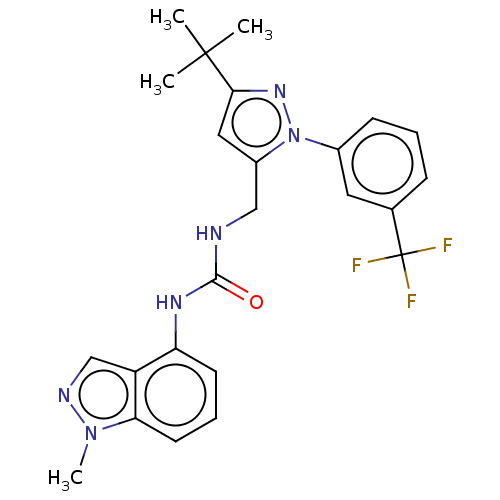 (CHEMBL4756342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553836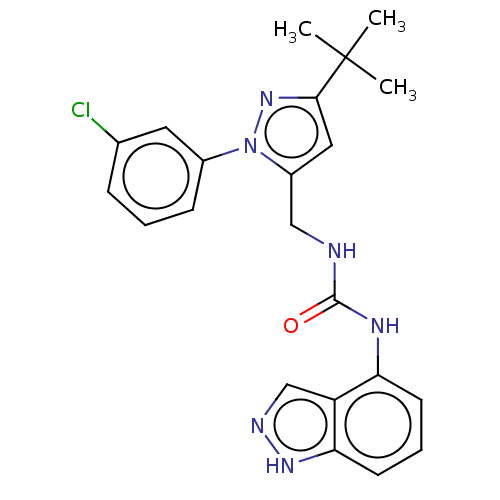 (CHEMBL4753881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342117 (N-((2-(tert-butyl)-4-(3-chlorophenyl)thiazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553843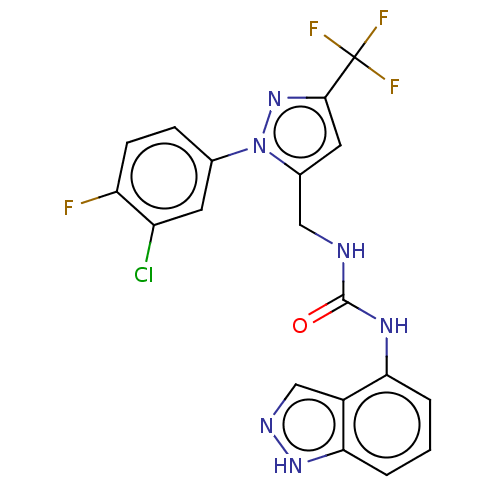 (CHEMBL4763915) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342121 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)oxazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM352414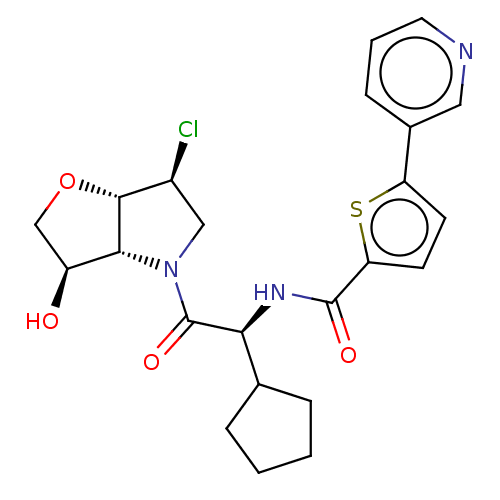 (Nó((S)-2-((3aS,6S,6aS)-6-chloro-3-oxotetrahydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRÜNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL, CatB) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a... | US Patent US9802947 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342122 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342119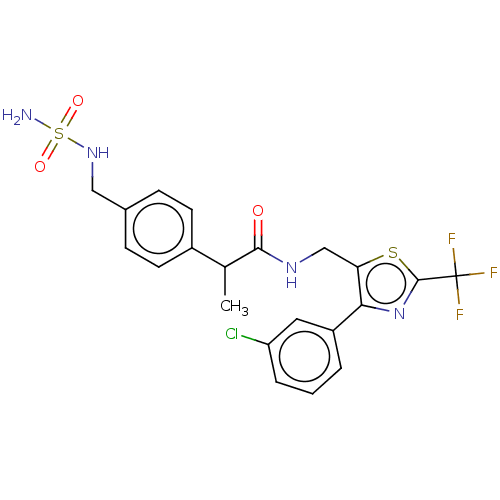 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM352420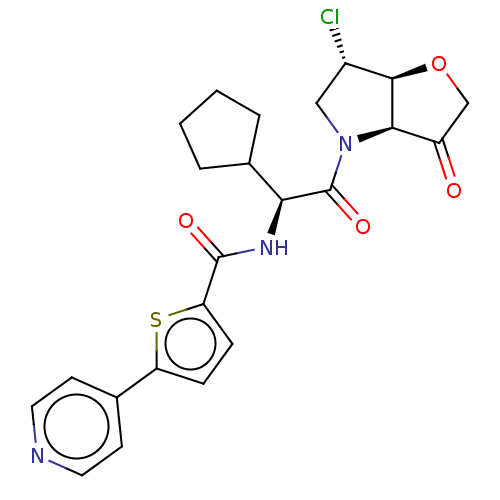 (Nó((S)-2-((3aS,6S,6aS)-6-chloro-3-oxotetrahydro-2H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRÜNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL, CatB) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a... | US Patent US9802947 (2017) BindingDB Entry DOI: 10.7270/Q2HX1FT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176571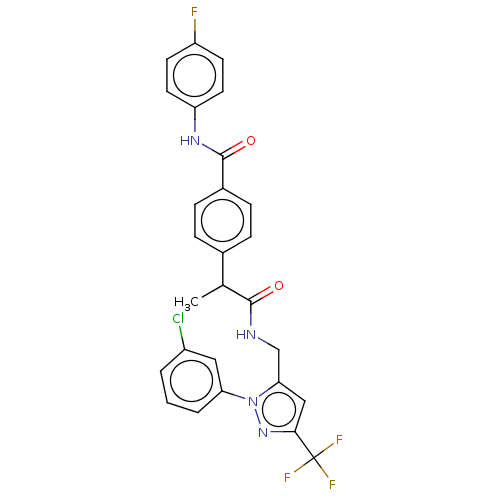 (US9120756, 40) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553833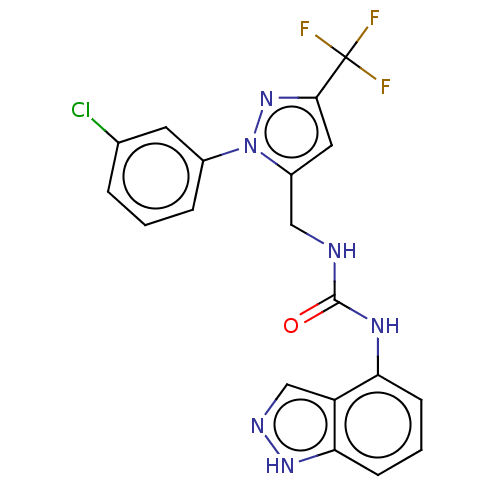 (CHEMBL4752959) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM331621 (N-((S)-2-((3aS,6S,6aS)-6-chloro-3- oxotetrahydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GRUNENTHAL GMBH US Patent | Assay Description Recombinant human cathepsins (CatS, CatK, CatL) were purchased from a Enzo Life Sciences. All assays were carried out in 96-well format using a buffe... | US Patent US9725459 (2017) BindingDB Entry DOI: 10.7270/Q2JH3P8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM125072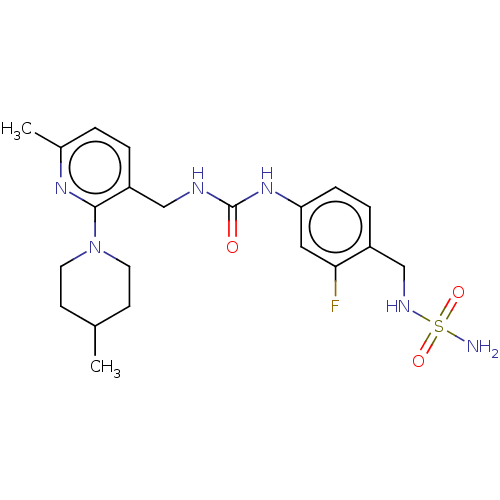 (US8765733, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of 2 substance additions. First the compounds to be tested (10 μM) are pipetted onto the cells and the Ca2+ influx i... | US Patent US8765733 (2014) BindingDB Entry DOI: 10.7270/Q2BZ64QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM125070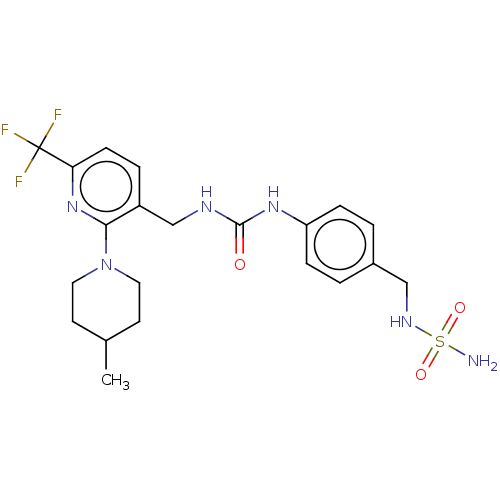 (US8765733, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of 2 substance additions. First the compounds to be tested (10 μM) are pipetted onto the cells and the Ca2+ influx i... | US Patent US8765733 (2014) BindingDB Entry DOI: 10.7270/Q2BZ64QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM50553867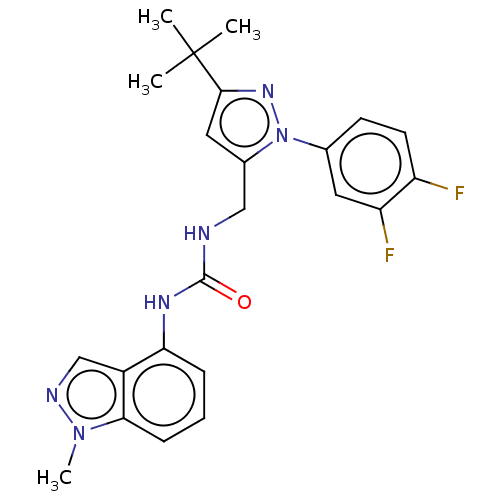 (CHEMBL4790987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human TRPV1 expressed in CHO-K1 cells assessed as inhibition of capsaicin-induced Ca2+ response treated with compound 6 mins p... | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127548 BindingDB Entry DOI: 10.7270/Q2VD733R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342120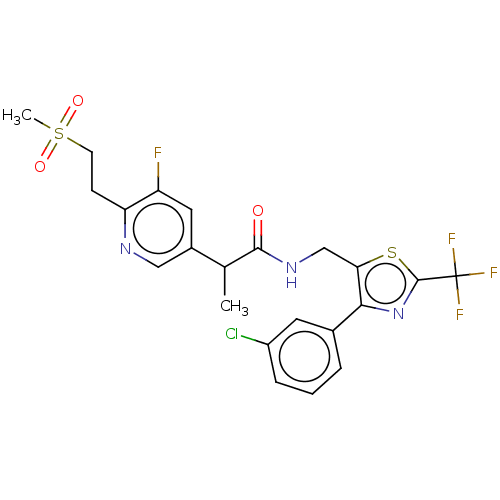 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176565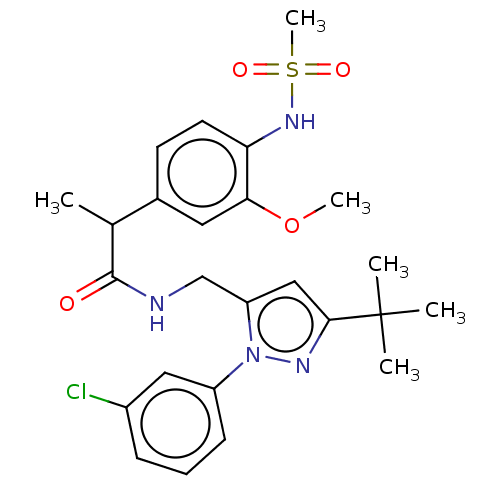 (US9120756, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176567 (US9120756, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 456 total ) | Next | Last >> |