 Found 299 hits with Last Name = 'li' and Initial = 'yp'
Found 299 hits with Last Name = 'li' and Initial = 'yp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Yes |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086454
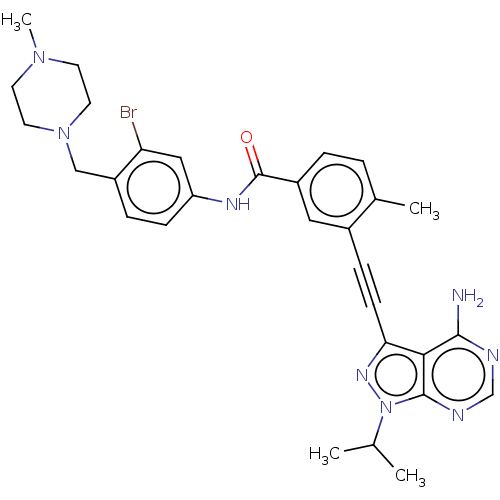
(CHEMBL3425518)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Br)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33BrN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Yes
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant YES using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086451
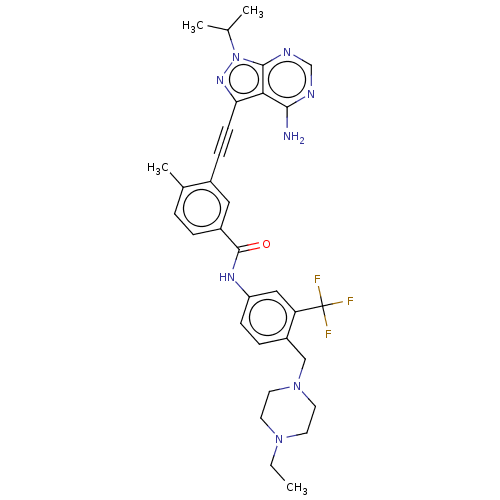
(CHEMBL3426222)Show SMILES CCN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C(C)C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H35F3N8O/c1-5-41-12-14-42(15-13-41)18-24-8-10-25(17-26(24)32(33,34)35)39-31(44)23-7-6-21(4)22(16-23)9-11-27-28-29(36)37-19-38-30(28)43(40-27)20(2)3/h6-8,10,16-17,19-20H,5,12-15,18H2,1-4H3,(H,39,44)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086455
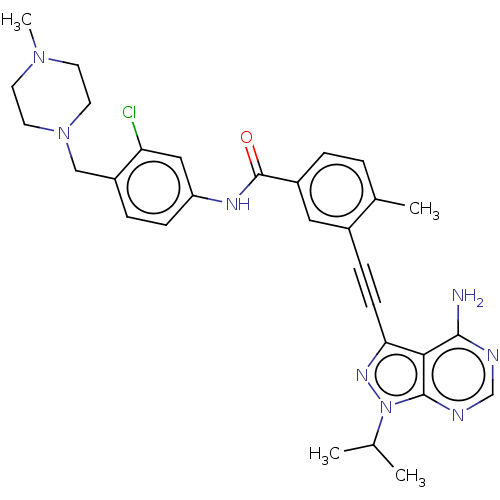
(CHEMBL3426219)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(Cl)c2)c2c(N)ncnc12 Show InChI InChI=1S/C30H33ClN8O/c1-19(2)39-29-27(28(32)33-18-34-29)26(36-39)10-8-21-15-22(6-5-20(21)3)30(40)35-24-9-7-23(25(31)16-24)17-38-13-11-37(4)12-14-38/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,35,40)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086457
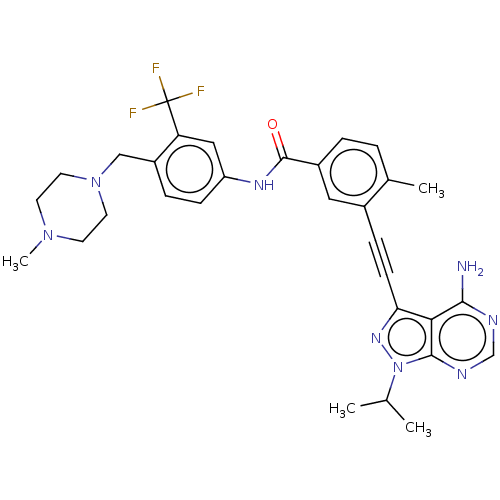
(CHEMBL3426217)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C31H33F3N8O/c1-19(2)42-29-27(28(35)36-18-37-29)26(39-42)10-8-21-15-22(6-5-20(21)3)30(43)38-24-9-7-23(25(16-24)31(32,33)34)17-41-13-11-40(4)12-14-41/h5-7,9,15-16,18-19H,11-14,17H2,1-4H3,(H,38,43)(H2,35,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333767

(CHEMBL1644288 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...)Show SMILES COc1cc(CNCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C29H39N3O3/c1-33-26-18-21(19-27(34-2)29(26)35-3)20-30-16-10-4-5-11-17-31-28-22-12-6-8-14-24(22)32-25-15-9-7-13-23(25)28/h6,8,12,14,18-19,30H,4-5,7,9-11,13,15-17,20H2,1-3H3,(H,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333763
(CHEMBL1644292 | N1-(3,4-Dimethoxybenzyl)-N6-(1,2,3...)Show SMILES COc1ccc(CNCCCCCCNc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C28H37N3O2/c1-32-26-16-15-21(19-27(26)33-2)20-29-17-9-3-4-10-18-30-28-22-11-5-7-13-24(22)31-25-14-8-6-12-23(25)28/h5,7,11,13,15-16,19,29H,3-4,6,8-10,12,14,17-18,20H2,1-2H3,(H,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant Src using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086453

(CHEMBL3426220 | US10266537, Compound 14)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2cccc(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C25H21F3N6O/c1-14(2)34-23-21(22(29)30-13-31-23)20(33-34)10-9-16-11-17(8-7-15(16)3)24(35)32-19-6-4-5-18(12-19)25(26,27)28/h4-8,11-14H,1-3H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ABL T315I mutant (27 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase HCK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333765

(CHEMBL1644290 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...)Show SMILES COc1cc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C31H43N3O3/c1-35-28-20-23(21-29(36-2)31(28)37-3)22-32-18-12-6-4-5-7-13-19-33-30-24-14-8-10-16-26(24)34-27-17-11-9-15-25(27)30/h8,10,14,16,20-21,32H,4-7,9,11-13,15,17-19,22H2,1-3H3,(H,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345200

(2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES COc1cc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C29H39N3O2/c1-34-28-20-22(16-17-27(28)33)21-30-18-10-4-2-3-5-11-19-31-29-23-12-6-8-14-25(23)32-26-15-9-7-13-24(26)29/h6,8,12,14,16-17,20,30,33H,2-5,7,9-11,13,15,18-19,21H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345205

(CHEMBL1783241 | N1-(Benzo[d][1,3]dioxol-5-ylmethyl...)Show SMILES C(CCCCNc1c2CCCCc2nc2ccccc12)CCCNCc1ccc2OCOc2c1 Show InChI InChI=1S/C29H37N3O2/c1(3-9-17-30-20-22-15-16-27-28(19-22)34-21-33-27)2-4-10-18-31-29-23-11-5-7-13-25(23)32-26-14-8-6-12-24(26)29/h5,7,11,13,15-16,19,30H,1-4,6,8-10,12,14,17-18,20-21H2,(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.44 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333766

(CHEMBL1644289 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...)Show SMILES COc1cc(CNCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C30H41N3O3/c1-34-27-19-22(20-28(35-2)30(27)36-3)21-31-17-11-5-4-6-12-18-32-29-23-13-7-9-15-25(23)33-26-16-10-8-14-24(26)29/h7,9,13,15,19-20,31H,4-6,8,10-12,14,16-18,21H2,1-3H3,(H,32,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Pancreatic triacylglycerol lipase
(Sus scrofa (Pig)) | BDBM24567

((2S)-1-[(2S,3S)-3-hexyl-4-oxooxetan-2-yl]tridecan-...)Show SMILES CCCCCCCCCCC[C@@H](C[C@@H]1OC(=O)[C@H]1CCCCCC)OC(=O)[C@H](CC(C)C)NC=O |r| Show InChI InChI=1S/C29H53NO5/c1-5-7-9-11-12-13-14-15-16-18-24(34-29(33)26(30-22-31)20-23(3)4)21-27-25(28(32)35-27)19-17-10-8-6-2/h22-27H,5-21H2,1-4H3,(H,30,31)/t24-,25-,26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic lipase using p-NPB as substrate pretreated for 15 mins followed by substrate addition measured after 15 mins by spec... |
J Nat Prod 81: 1098-1102 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00017
BindingDB Entry DOI: 10.7270/Q2930WVD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant RET (658 to end residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL2
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant ARG (38 to end residues) using EAIYAAPFAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radio... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometri... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086450
(CHEMBL3426223)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(CCO)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C32H35F3N8O2/c1-20(2)43-30-28(29(36)37-19-38-30)27(40-43)9-7-22-16-23(5-4-21(22)3)31(45)39-25-8-6-24(26(17-25)32(33,34)35)18-42-12-10-41(11-13-42)14-15-44/h4-6,8,16-17,19-20,44H,10-15,18H2,1-3H3,(H,39,45)(H2,36,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086456

(CHEMBL3426218 | US10266537, Compound 17)Show SMILES CC(C)n1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)cc2)c2c(N)ncnc12 Show InChI InChI=1S/C30H34N8O/c1-20(2)38-29-27(28(31)32-19-33-29)26(35-38)12-9-23-17-24(8-5-21(23)3)30(39)34-25-10-6-22(7-11-25)18-37-15-13-36(4)14-16-37/h5-8,10-11,17,19-20H,13-16,18H2,1-4H3,(H,34,39)(H2,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345201
(2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...)Show SMILES COc1cc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1O Show InChI InChI=1S/C30H41N3O3/c1-35-27-19-22(20-28(36-2)30(27)34)21-31-17-11-5-3-4-6-12-18-32-29-23-13-7-9-15-25(23)33-26-16-10-8-14-24(26)29/h7,9,13,15,19-20,31,34H,3-6,8,10-12,14,16-18,21H2,1-2H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.08 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50345200

(2-Methoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-ylami...)Show SMILES COc1cc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C29H39N3O2/c1-34-28-20-22(16-17-27(28)33)21-30-18-10-4-2-3-5-11-19-31-29-23-12-6-8-14-25(23)32-26-15-9-7-13-24(26)29/h6,8,12,14,16-17,20,30,33H,2-5,7,9-11,13,15,18-19,21H2,1H3,(H,31,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50345201

(2,6-Dimethoxy-4-((8-(1,2,3,4-tetrahydroacridin-9-y...)Show SMILES COc1cc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1O Show InChI InChI=1S/C30H41N3O3/c1-35-27-19-22(20-28(36-2)30(27)34)21-31-17-11-5-3-4-6-12-18-32-29-23-13-7-9-15-25(23)33-26-16-10-8-14-24(26)29/h7,9,13,15,19-20,31,34H,3-6,8,10-12,14,16-18,21H2,1-2H3,(H,32,33) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345206

(CHEMBL1783242 | N1-(Pyridin-4-ylmethyl)-N8-(1,2,3,...)Show InChI InChI=1S/C27H36N4/c1(3-9-17-29-21-22-15-19-28-20-16-22)2-4-10-18-30-27-23-11-5-7-13-25(23)31-26-14-8-6-12-24(26)27/h5,7,11,13,15-16,19-20,29H,1-4,6,8-10,12,14,17-18,21H2,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50345206

(CHEMBL1783242 | N1-(Pyridin-4-ylmethyl)-N8-(1,2,3,...)Show InChI InChI=1S/C27H36N4/c1(3-9-17-29-21-22-15-19-28-20-16-22)2-4-10-18-30-27-23-11-5-7-13-25(23)31-26-14-8-6-12-24(26)27/h5,7,11,13,15-16,19-20,29H,1-4,6,8-10,12,14,17-18,21H2,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333764

(CHEMBL1644291 | N1-(1,2,3,4-Tetrahydroacridin-9-yl...)Show SMILES COc1cc(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc(OC)c1OC Show InChI InChI=1S/C32H45N3O3/c1-36-29-21-24(22-30(37-2)32(29)38-3)23-33-19-13-7-5-4-6-8-14-20-34-31-25-15-9-11-17-27(25)35-28-18-12-10-16-26(28)31/h9,11,15,17,21-22,33H,4-8,10,12-14,16,18-20,23H2,1-3H3,(H,34,35) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345204
(CHEMBL1783240 | N1-(4-Methoxybenzyl)-N8-(1,2,3,4-t...)Show SMILES COc1ccc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc1 Show InChI InChI=1S/C29H39N3O/c1-33-24-18-16-23(17-19-24)22-30-20-10-4-2-3-5-11-21-31-29-25-12-6-8-14-27(25)32-28-15-9-7-13-26(28)29/h6,8,12,14,16-19,30H,2-5,7,9-11,13,15,20-22H2,1H3,(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345202
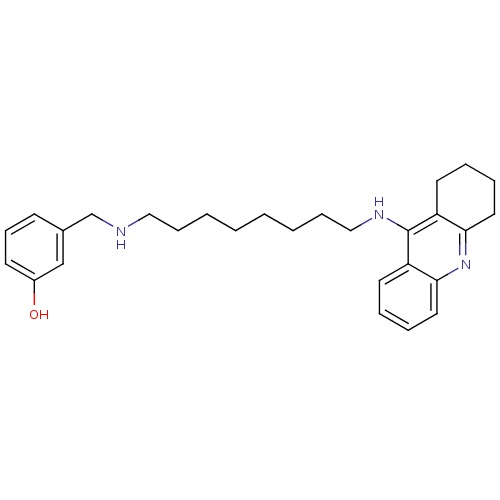
(3-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show InChI InChI=1S/C28H37N3O/c32-23-13-11-12-22(20-23)21-29-18-9-3-1-2-4-10-19-30-28-24-14-5-7-16-26(24)31-27-17-8-6-15-25(27)28/h5,7,11-14,16,20,29,32H,1-4,6,8-10,15,17-19,21H2,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50086441

(CHEMBL3426225 | US10266537, Compound 3)Show SMILES CCn1nc(C#Cc2cc(ccc2C)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C30H31F3N8O/c1-4-41-28-26(27(34)35-18-36-28)25(38-41)10-8-20-15-21(6-5-19(20)2)29(42)37-23-9-7-22(24(16-23)30(31,32)33)17-40-13-11-39(3)12-14-40/h5-7,9,15-16,18H,4,11-14,17H2,1-3H3,(H,37,42)(H2,34,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Fyn |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086586

(CHEMBL3426234 | US10266537, Compound 29)Show SMILES CCn1nc(C#Cc2cccc(c2)C(=O)Nc2ccc(CN3CCN(C)CC3)c(c2)C(F)(F)F)c2c(N)ncnc12 Show InChI InChI=1S/C29H29F3N8O/c1-3-40-27-25(26(33)34-18-35-27)24(37-40)10-7-19-5-4-6-20(15-19)28(41)36-22-9-8-21(23(16-22)29(30,31)32)17-39-13-11-38(2)12-14-39/h4-6,8-9,15-16,18H,3,11-14,17H2,1-2H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333771

(CHEMBL1644284 | N1-((7-Methoxybenzo[d][1,3]dioxol-...)Show SMILES COc1cc(CNCCCCCCNc2c3CCCCc3nc3ccccc23)cc2OCOc12 Show InChI InChI=1S/C28H35N3O3/c1-32-25-16-20(17-26-28(25)34-19-33-26)18-29-14-8-2-3-9-15-30-27-21-10-4-6-12-23(21)31-24-13-7-5-11-22(24)27/h4,6,10,12,16-17,29H,2-3,5,7-9,11,13-15,18-19H2,1H3,(H,30,31) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345199
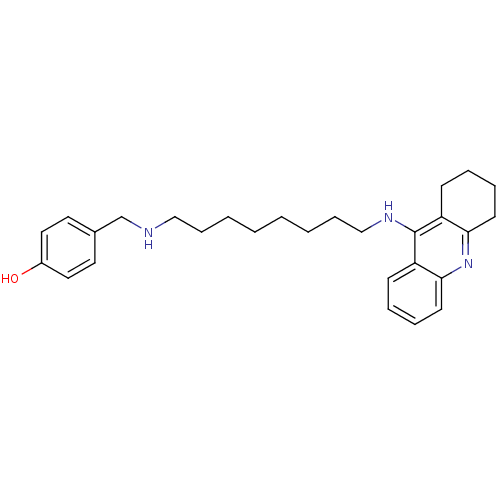
(4-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show InChI InChI=1S/C28H37N3O/c32-23-17-15-22(16-18-23)21-29-19-9-3-1-2-4-10-20-30-28-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)28/h5,7,11,13,15-18,29,32H,1-4,6,8-10,12,14,19-21H2,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333762
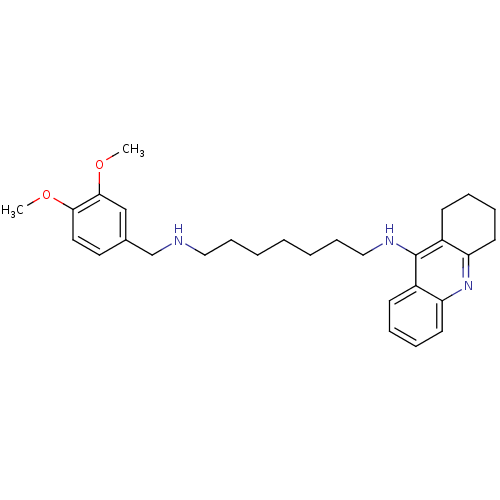
(CHEMBL1644293 | N1-(3,4-Dimethoxybenzyl)-N7-(1,2,3...)Show SMILES COc1ccc(CNCCCCCCCNc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C29H39N3O2/c1-33-27-17-16-22(20-28(27)34-2)21-30-18-10-4-3-5-11-19-31-29-23-12-6-8-14-25(23)32-26-15-9-7-13-24(26)29/h6,8,12,14,16-17,20,30H,3-5,7,9-11,13,15,18-19,21H2,1-2H3,(H,31,32) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333761
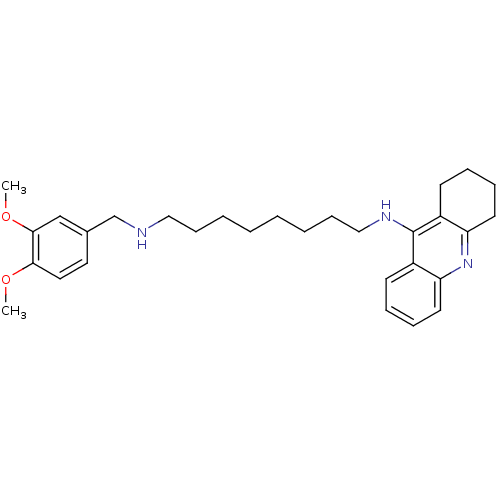
(CHEMBL1644294 | N1-(3,4-Dimethoxybenzyl)-N8-(1,2,3...)Show SMILES COc1ccc(CNCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C30H41N3O2/c1-34-28-18-17-23(21-29(28)35-2)22-31-19-11-5-3-4-6-12-20-32-30-24-13-7-9-15-26(24)33-27-16-10-8-14-25(27)30/h7,9,13,15,17-18,21,31H,3-6,8,10-12,14,16,19-20,22H2,1-2H3,(H,32,33) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50333760

(CHEMBL1644295 | N1-(3,4-Dimethoxybenzyl)-N9-(1,2,3...)Show SMILES COc1ccc(CNCCCCCCCCCNc2c3CCCCc3nc3ccccc23)cc1OC Show InChI InChI=1S/C31H43N3O2/c1-35-29-19-18-24(22-30(29)36-2)23-32-20-12-6-4-3-5-7-13-21-33-31-25-14-8-10-16-27(25)34-28-17-11-9-15-26(28)31/h8,10,14,16,18-19,22,32H,3-7,9,11-13,15,17,20-21,23H2,1-2H3,(H,33,34) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.67 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine BChE by Ellman's method |
Bioorg Med Chem 19: 763-70 (2011)
Article DOI: 10.1016/j.bmc.2010.12.022
BindingDB Entry DOI: 10.7270/Q2T43TB0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full length recombinant FYN using Cdc2 peptide as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric sc... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant TXK (256 to end residues) using GEEPLYWSFPAKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ra... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50345205

(CHEMBL1783241 | N1-(Benzo[d][1,3]dioxol-5-ylmethyl...)Show SMILES C(CCCCNc1c2CCCCc2nc2ccccc12)CCCNCc1ccc2OCOc2c1 Show InChI InChI=1S/C29H37N3O2/c1(3-9-17-30-20-22-15-16-27-28(19-22)34-21-33-27)2-4-10-18-31-29-23-11-5-7-13-25(23)32-26-14-8-6-12-24(26)29/h5,7,11,13,15-16,19,30H,1-4,6,8-10,12,14,17-18,20-21H2,(H,31,32) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086449

(CHEMBL3426224 | US10266537, Compound 8)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn(C)c4ncnc(N)c34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H29F3N8O/c1-18-4-5-20(14-19(18)7-9-24-25-26(33)34-17-35-27(25)39(3)37-24)28(41)36-22-8-6-21(23(15-22)29(30,31)32)16-40-12-10-38(2)11-13-40/h4-6,8,14-15,17H,10-13,16H2,1-3H3,(H,36,41)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086445

(CHEMBL3426229 | US10266537, Compound 21)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50086444

(CHEMBL3426230 | US10266537, Compound 20)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3nn([C@@H]4CCOC4)c4ncnc(N)c34)cc2C(F)(F)F)CC1 |r| Show InChI InChI=1S/C32H33F3N8O2/c1-20-3-4-22(15-21(20)6-8-27-28-29(36)37-19-38-30(28)43(40-27)25-9-14-45-18-25)31(44)39-24-7-5-23(26(16-24)32(33,34)35)17-42-12-10-41(2)11-13-42/h3-5,7,15-16,19,25H,9-14,17-18H2,1-2H3,(H,39,44)(H2,36,37,38)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sichuan University
Curated by ChEMBL
| Assay Description
Inhibition of human Src |
J Med Chem 58: 3957-74 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00270
BindingDB Entry DOI: 10.7270/Q2J38V91 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM378885

(US10266537, Compound 93)Show SMILES Cc1ccc(NC(=O)c2ccc(C)c(c2)C(F)(F)F)cc1C#Cc1nn([C@H]2CC[C@H](O)CC2)c2ncnc(N)c12 |r,wU:26.27,wD:29.31,(-5.46,5.4,;-3.98,5.8,;-3.58,7.29,;-2.09,7.69,;-1,6.6,;.49,7,;1.57,5.91,;1.18,4.42,;3.06,6.31,;3.46,7.79,;4.95,8.19,;6.04,7.1,;7.53,7.5,;5.64,5.62,;4.15,5.22,;6.73,4.53,;8.22,4.93,;6.33,3.04,;7.82,3.44,;-1.4,5.11,;-2.89,4.71,;-3.29,3.22,;-3.68,1.74,;-4.08,.25,;-3.18,-1,;-4.08,-2.24,;-3.68,-3.73,;-2.2,-4.13,;-1.8,-5.62,;-2.89,-6.7,;-2.49,-8.19,;-4.38,-6.31,;-4.77,-4.82,;-5.55,-1.77,;-6.88,-2.54,;-8.22,-1.77,;-8.22,-.23,;-6.88,.54,;-6.88,2.08,;-5.55,-.23,)| Show InChI InChI=1S/C29H27F3N6O2/c1-16-4-7-20(36-28(40)19-5-3-17(2)23(14-19)29(30,31)32)13-18(16)6-12-24-25-26(33)34-15-35-27(25)38(37-24)21-8-10-22(39)11-9-21/h3-5,7,13-15,21-22,39H,8-11H2,1-2H3,(H,36,40)(H2,33,34,35)/t21-,22- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant FGFR1 (456 to 765 residues) using KKKSPGEYVNIEFG as substrate incubated for 40 mins in presence of [gamma33P-ATP] by ... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2M90DC6 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50345199

(4-((8-(1,2,3,4-Tetrahydroacridin-9-ylamino)octylam...)Show InChI InChI=1S/C28H37N3O/c32-23-17-15-22(16-18-23)21-29-19-9-3-1-2-4-10-20-30-28-24-11-5-7-13-26(24)31-27-14-8-6-12-25(27)28/h5,7,11,13,15-18,29,32H,1-4,6,8-10,12,14,19-21H2,(H,30,31) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE assessed as inhibition of acetylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM50345197
(4-((6-(1,2,3,4-Tetrahydroacridin-9-ylamino)hexylam...)Show InChI InChI=1S/C26H33N3O/c30-21-15-13-20(14-16-21)19-27-17-7-1-2-8-18-28-26-22-9-3-5-11-24(22)29-25-12-6-4-10-23(25)26/h3,5,9,11,13-16,27,30H,1-2,4,6-8,10,12,17-19H2,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BuChE assessed as inhibition of butylthiocholine chloride substrate hydrolysis incubated for 15 mins before substrate addi... |
Eur J Med Chem 46: 2609-16 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.058
BindingDB Entry DOI: 10.7270/Q2BV7GZB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data