

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50257088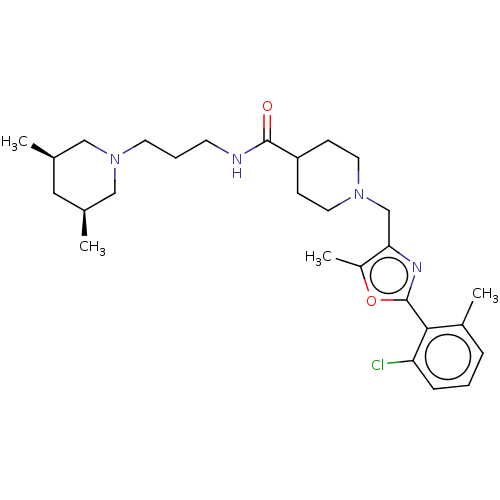 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 443 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Cimetidine from human H2 receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human alpha2C receptor expressed in MDCK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from KOR receptor (unknown origin) expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Nisoxetine from human NET receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Rauwolscine from human alpha2A receptor expressed in MDCK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]QNB from human M5 receptor expressed in CHO cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50257088 (CHEMBL4066784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Pentazocine from guinea pig Sigma1 receptor after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50257088 (CHEMBL4066784) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health , 10 Center Drive, Bethesda, Maryland 20892-1800, United States. Curated by ChEMBL | Assay Description Displacement of [3H]Citalopram from human SERT receptor expressed in HEK cell membranes after 90 mins by scintillation counting method | J Med Chem 60: 6364-6383 (2017) Article DOI: 10.1021/acs.jmedchem.7b00561 BindingDB Entry DOI: 10.7270/Q2DR2XXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50041407 (CHEMBL3138697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of CDC25B (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041384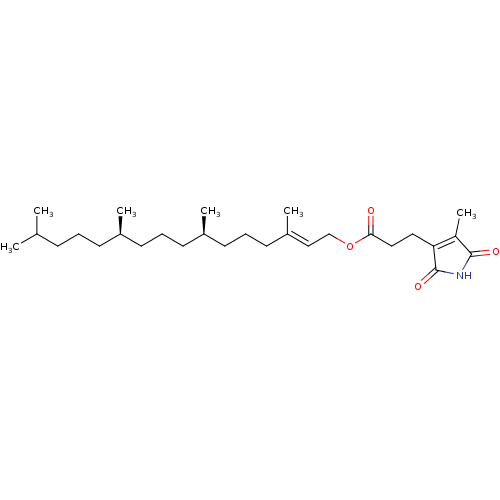 (CHEMBL3356396) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041407 (CHEMBL3138697) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041411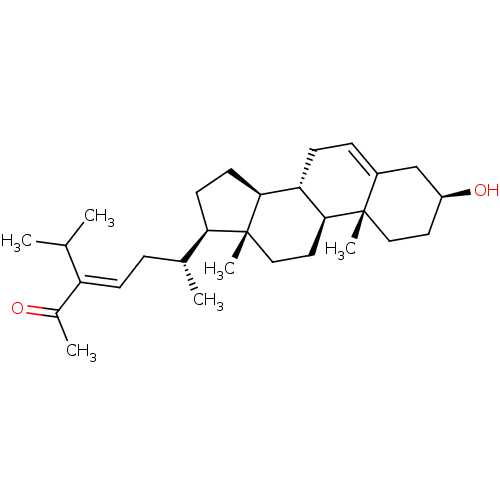 (CHEMBL3356399) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041386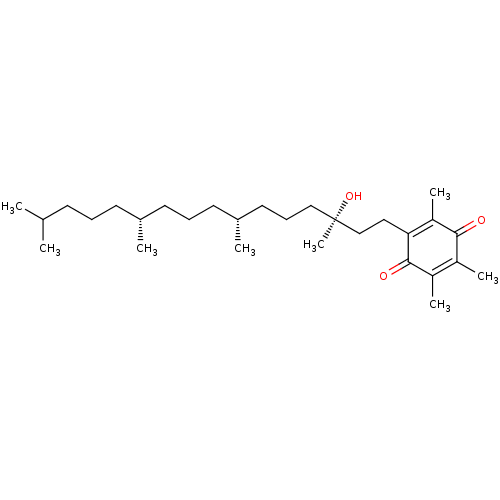 (Alpha-Tocopherylquinone | CHEMBL1223852) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50041407 (CHEMBL3138697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of TC-PTP (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041410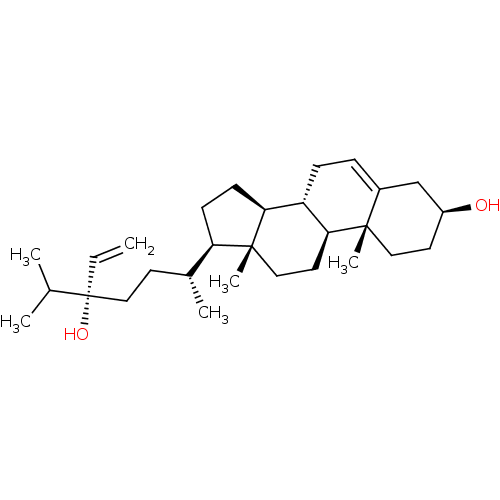 (CHEMBL524098) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041412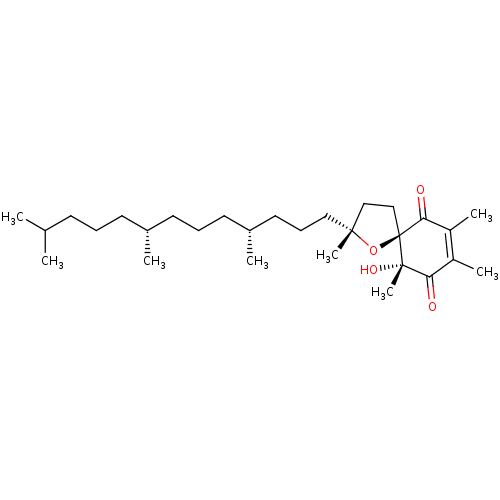 (CHEMBL3356398) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50041384 (CHEMBL3356396) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of TC-PTP (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase F (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of LAR (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of SHP2 (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 6 (Homo sapiens (Human)) | BDBM50391109 (CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of SHP1 (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50041386 (Alpha-Tocopherylquinone | CHEMBL1223852) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of CDC25B (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041288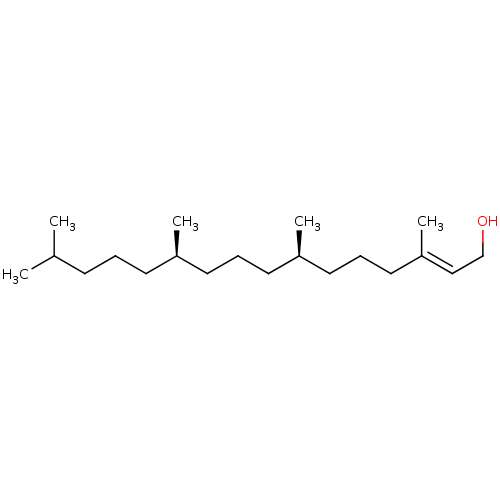 (CHEBI:17327 | Trans-Phytol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM50041386 (Alpha-Tocopherylquinone | CHEMBL1223852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of TC-PTP (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041409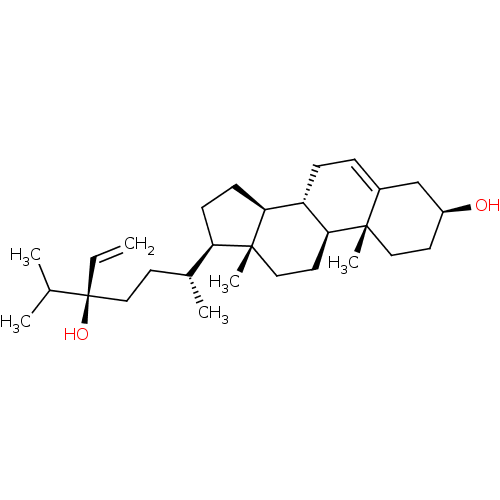 (CHEMBL489548) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50041384 (CHEMBL3356396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of CDC25B (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041408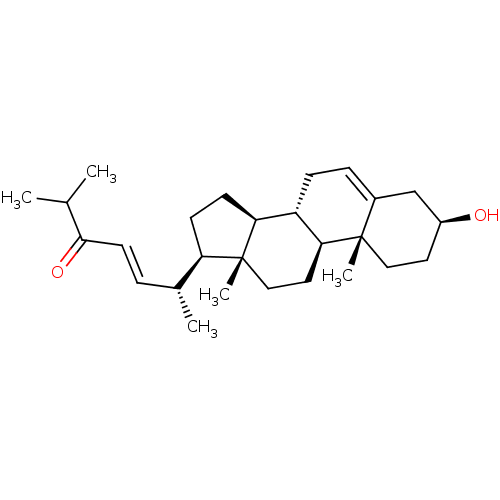 (CHEMBL3356400) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50041413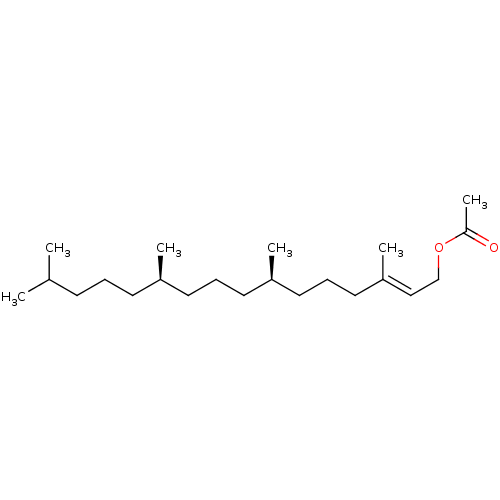 (CHEMBL3356397) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanchang University Curated by ChEMBL | Assay Description Inhibition of purified recombinant PTP1B catalytic domain (unknown origin) using pNPP as substrate by spectrophotometry | Bioorg Med Chem 23: 38-45 (2014) Article DOI: 10.1016/j.bmc.2014.11.031 BindingDB Entry DOI: 10.7270/Q20K2B5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||