

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497604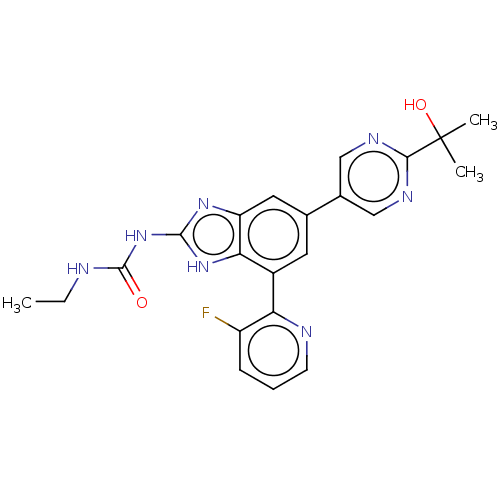 (CHEMBL3264033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497603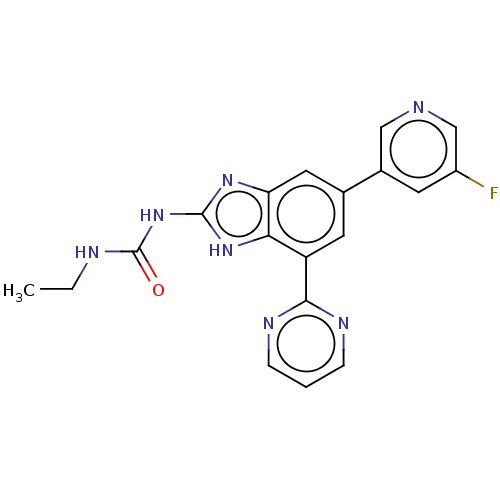 (CHEMBL3356986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50497602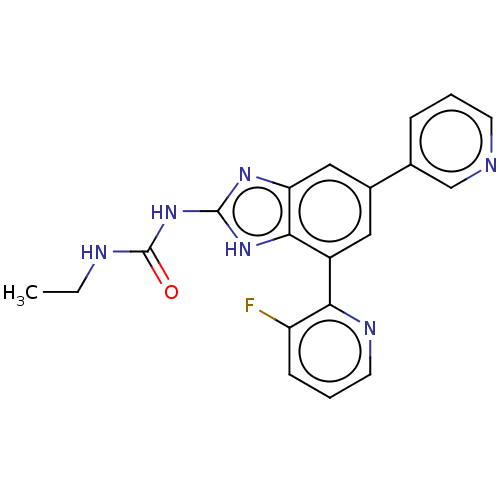 (CHEMBL222333 | VRT-752586) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of aureus Escherichia coli DNA gyrase A2B2 using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Staphylococcus aureus) | BDBM50393079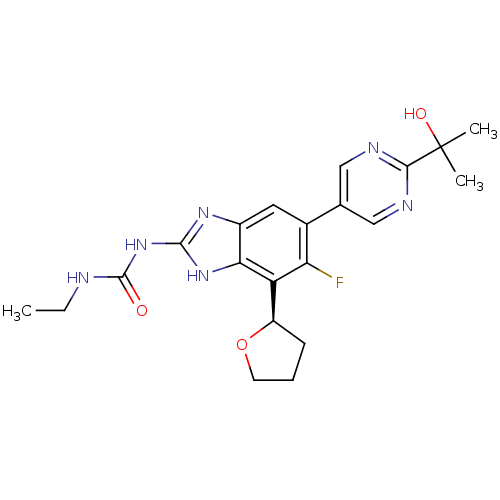 (CHEMBL2152855 | US9040542, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus DNA gyrase using pBR322 plasmid DNA as substrate by coupled enzyme reaction assay | J Med Chem 57: 8792-816 (2014) Article DOI: 10.1021/jm500563g BindingDB Entry DOI: 10.7270/Q2TF01BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343634 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343641 (CHEMBL1774964 | cis-4-((E)-4-((S)-4-methyl-1-oxo-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343632 (4-((E)-4-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343644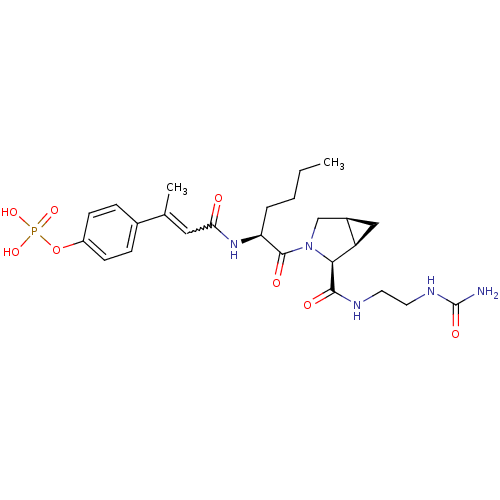 (CHEMBL1774967 | cis-4-((E)-4-oxo-4-((S)-1-oxo-1-((...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343633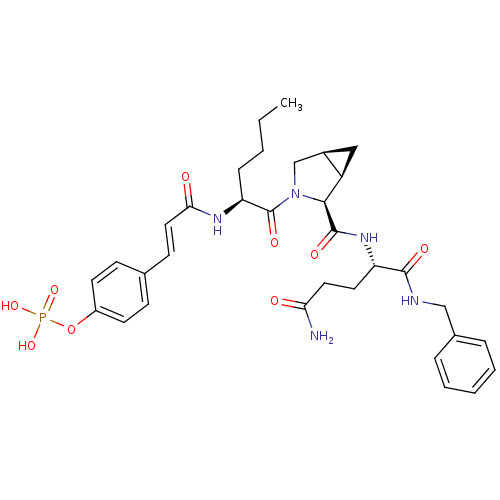 (4-((E)-3-((S)-1-((1R,2S,5S)-2-((S)-5-amino-1-(benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11233 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 53 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343635 (4-((E)-4-((3S,6S)-6-((S)-5-amino-1-(benzylamino)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11232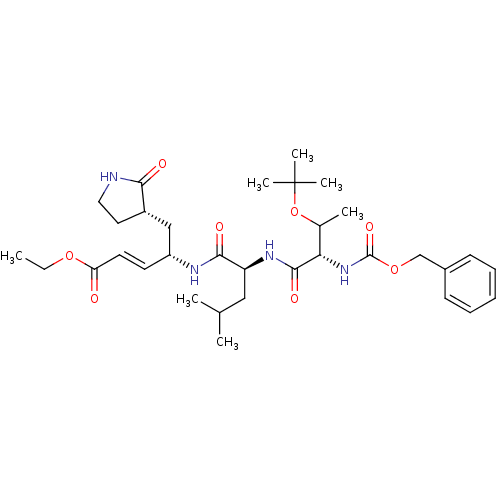 (N-[(benzyloxy)carbonyl]-O-(tert-butyl)-L-threonyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 58 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343642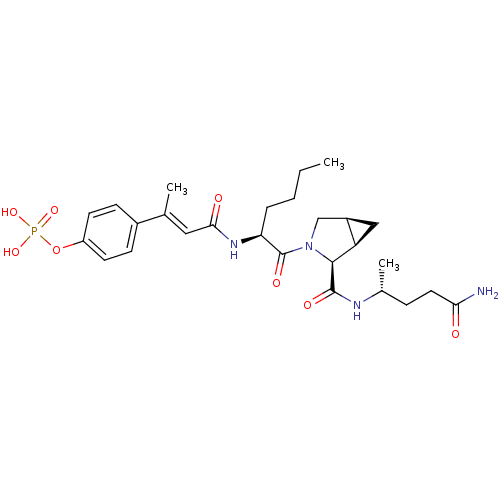 (CHEMBL1774965 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343631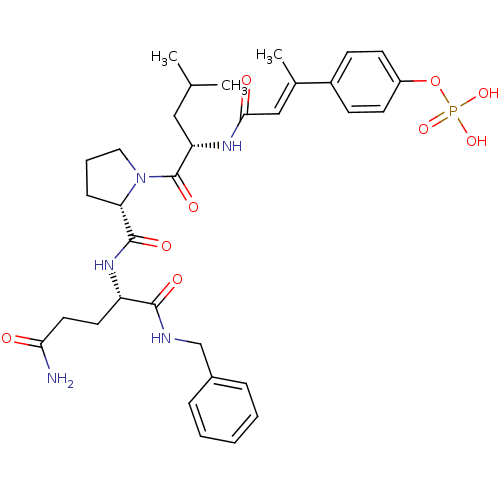 (4-((E)-4-((S)-1-((S)-2-((S)-5-amino-1-(benzylamino...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343639 (CHEMBL1774962 | cis-4-((E)-4-((S)-1-((1R,2S,5S)-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343638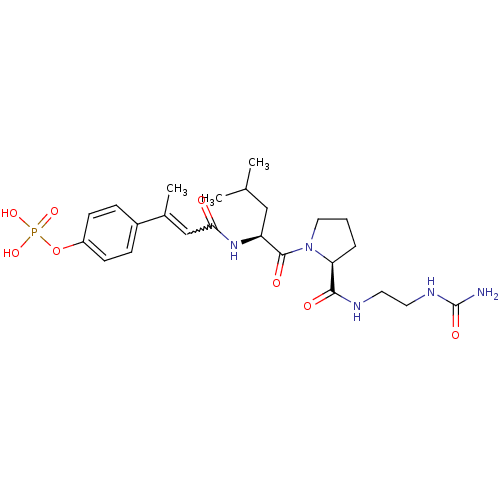 (4-((E)-4-((S)-4-methyl-1-oxo-1-((S)-2-(2-ureidoeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343645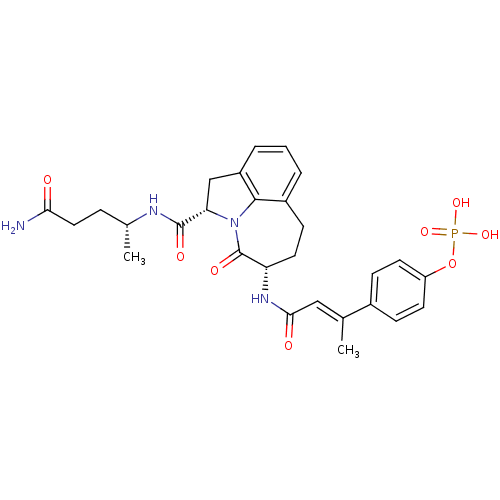 (4-((E)-4-((3S,6S)-6-((R)-5-amino-5-oxopentan-2-ylc...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343643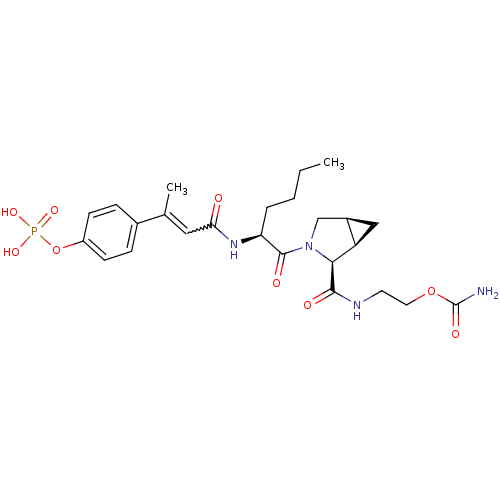 (CHEMBL1774966 | cis-2-((1R,2S,5S)-3-((S)-2-((E)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343636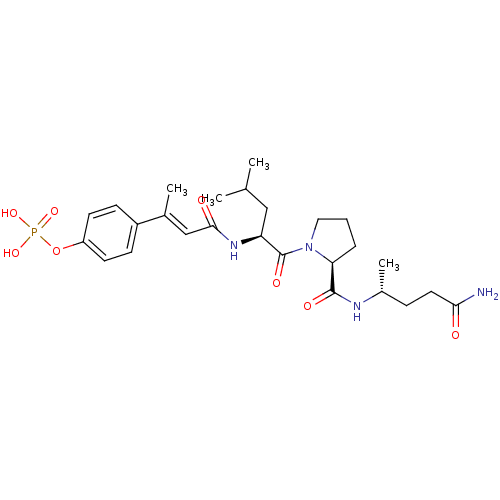 (4-((E)-4-((S)-1-((S)-2-((R)-5-amino-5-oxopentan-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343646 (2-((3S,6S)-4-oxo-3-((E)-3-(4-(phosphonooxy)phenyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50060080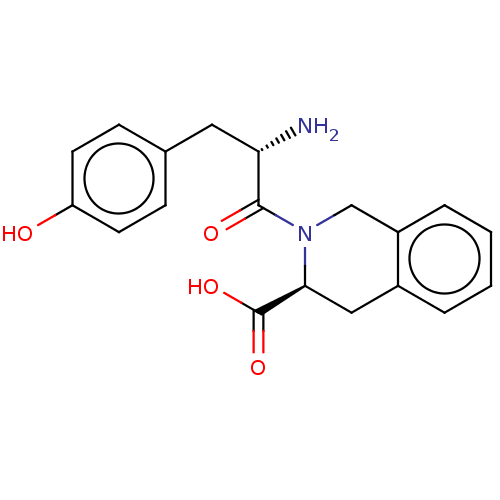 (2-({2-[(R)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the binding affinity towards delta-opioid receptor by displacement of [3H]p-CI-DPDPE radioligand from mouse vas defere... | Bioorg Med Chem Lett 7: 3049-3052 (1997) Article DOI: 10.1016/S0960-894X(97)10145-7 BindingDB Entry DOI: 10.7270/Q2H13211 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343640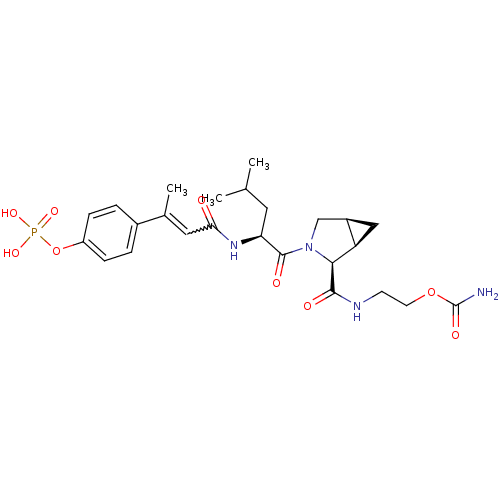 (CHEMBL1774963 | cis-2-((1R,2S,5S)-3-((S)-4-methyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 193 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343637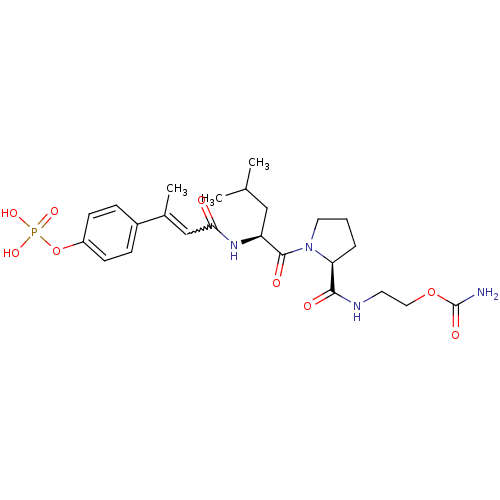 (2-((S)-1-((S)-4-methyl-2-((E)-3-(4-(phosphonooxy)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal transducer and activator of transcription 3 (Homo sapiens (Human)) | BDBM50343647 (4-((E)-4-oxo-4-((3S,6S)-4-oxo-6-(2-ureidoethylcarb...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas M. D. Anderson Cancer Center Curated by ChEMBL | Assay Description Binding affinity to SH2 domain of Stat3 by fluorescence polarization assay | J Med Chem 54: 3549-63 (2011) Article DOI: 10.1021/jm2000882 BindingDB Entry DOI: 10.7270/Q2G44QMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11231 (N-[(benzyloxy)carbonyl]-L-valyl-N1-((1S,2E)-4-etho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11230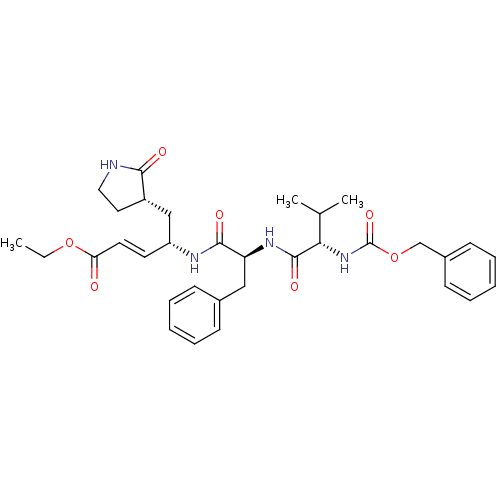 (AG7088 analogue 2d | CHEMBL277716 | N-[(benzyloxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.26E+3 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Human SARS coronavirus (SARS-CoV) (Severe acute re...) | BDBM11229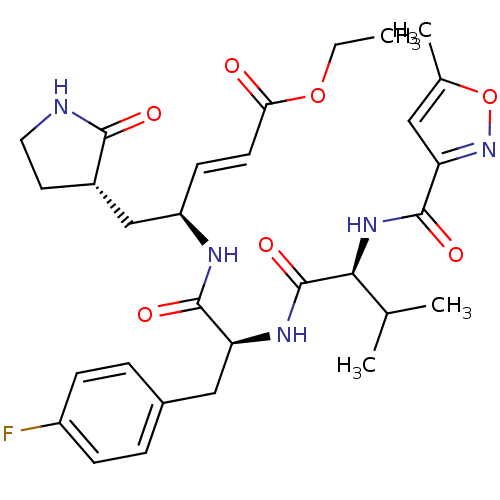 (AG7088 analogue 2a | CHEMBL20636 | N-[(5-methyliso...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+4 | >-28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
TaiGen Biotechnology Co. | Assay Description The effects of compound on enzyme activity were measured by using peptide cleavage assay. Cleavage products were resolved and analyzed with a reverse... | J Med Chem 49: 4971-80 (2006) Article DOI: 10.1021/jm0603926 BindingDB Entry DOI: 10.7270/Q24B2ZJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50060080 (2-({2-[(R)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was evaluated for the binding affinity towards mu-opioid receptor by displacement of [3H]DAMGO radioligand from guinea pig ileum | Bioorg Med Chem Lett 7: 3049-3052 (1997) Article DOI: 10.1016/S0960-894X(97)10145-7 BindingDB Entry DOI: 10.7270/Q2H13211 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581036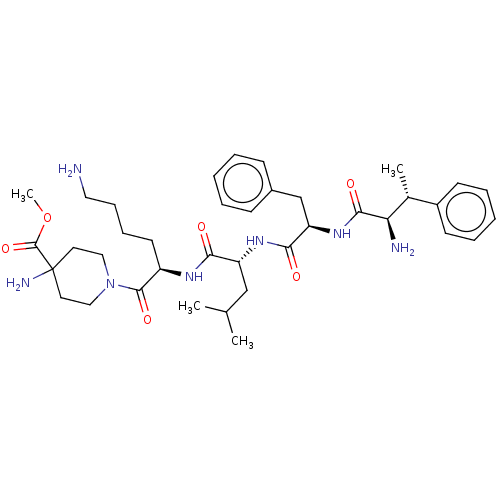 (RFCX0139-43 | US11492374, ID 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.157 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM581033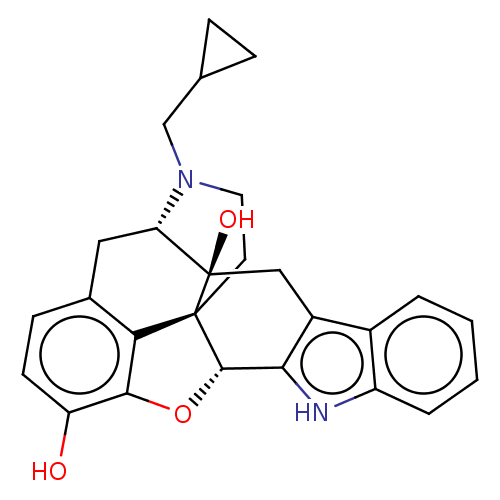 (US11492374, ID 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay | Eur J Med Chem 62: 597-604 (2013) Article DOI: 10.1016/j.ejmech.2013.01.030 BindingDB Entry DOI: 10.7270/Q2X92CNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581037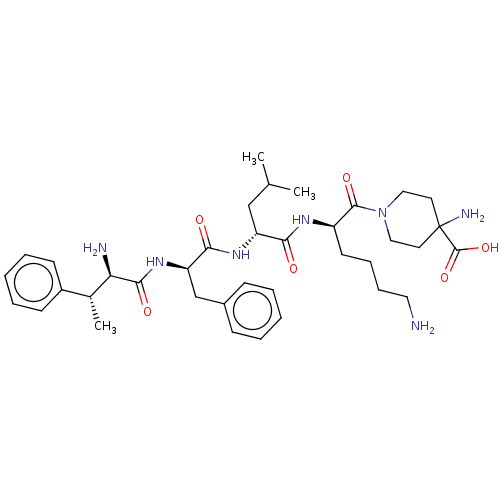 (RFCX0139-45 | US11492374, ID 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.253 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581034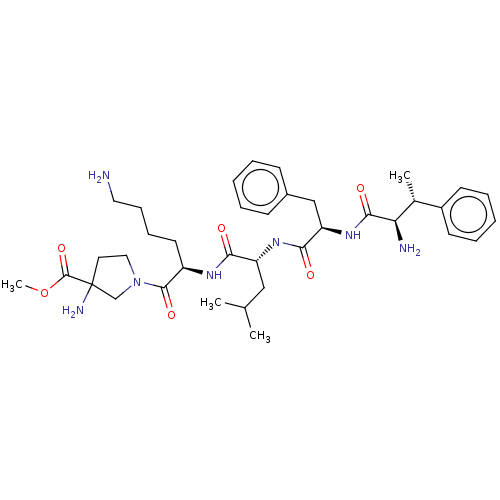 (US11492374, ID 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.255 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.294 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of full length carbonic anhydrase-2 in human erythrocytes | Bioorg Med Chem Lett 23: 3496-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.048 BindingDB Entry DOI: 10.7270/Q2GH9KFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039026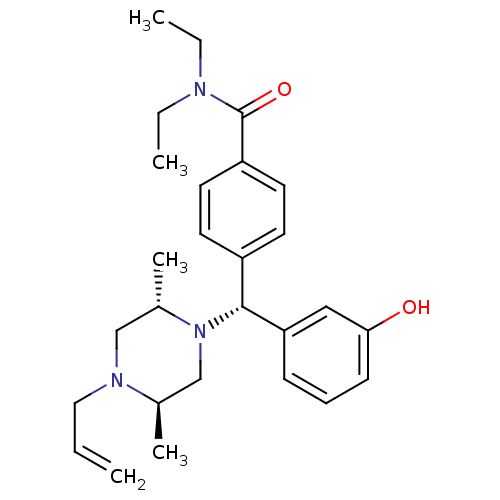 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM581035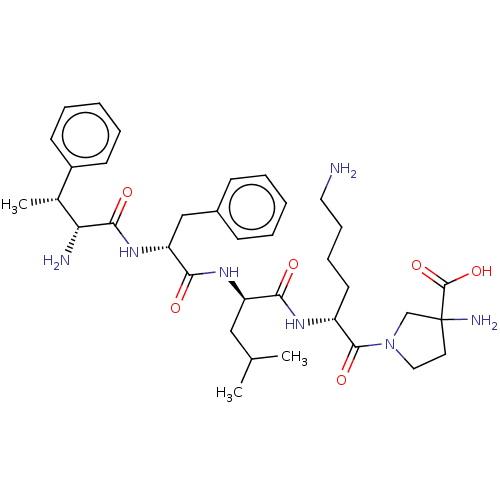 (US11492374, ID 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The measurement of opioid receptor binding affinity was conducted using a radioligand binding assay on the membranes prepared from HEK293 cells (huma... | Citation and Details BindingDB Entry DOI: 10.7270/Q26977FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50430551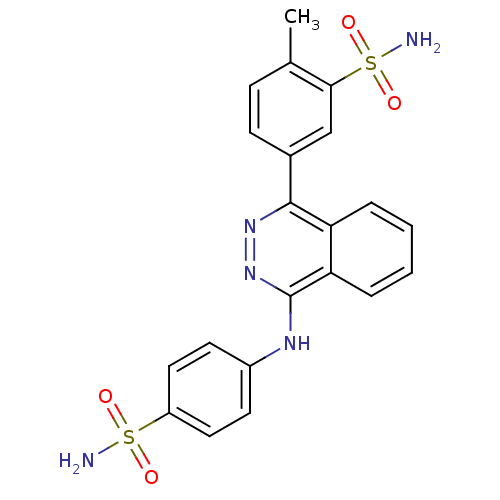 (CHEMBL2336905) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay | Eur J Med Chem 62: 597-604 (2013) Article DOI: 10.1016/j.ejmech.2013.01.030 BindingDB Entry DOI: 10.7270/Q2X92CNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) | Bioorg Med Chem Lett 23: 3496-9 (2013) Article DOI: 10.1016/j.bmcl.2013.04.048 BindingDB Entry DOI: 10.7270/Q2GH9KFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50610140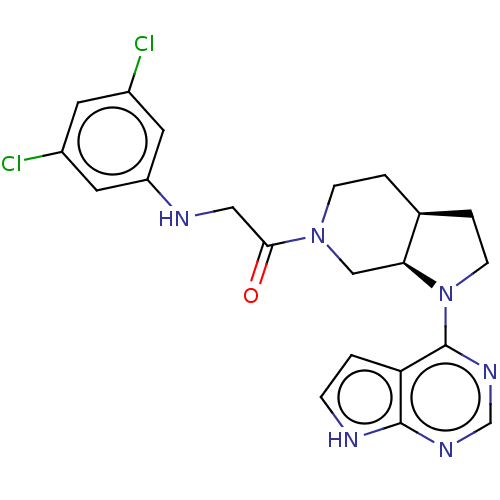 (CHEMBL5266817) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50610140 (CHEMBL5266817) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM418817 (N-methyl-4-({4-[({3- [methyl(methylsulfonyl)amino]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113573 BindingDB Entry DOI: 10.7270/Q2125XFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50068133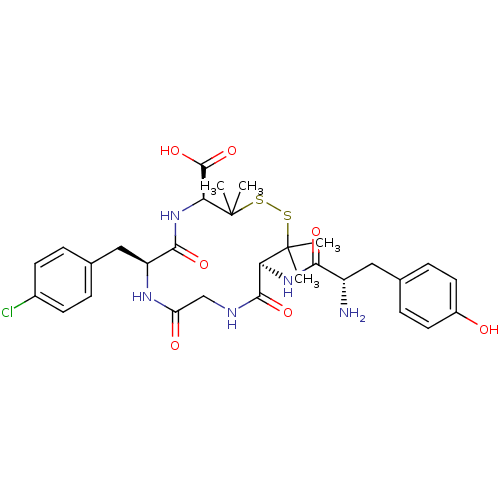 ((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against cloned human Opioid receptor delta 1 (wild-type,Wt) | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50068133 ((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50068133 ((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona Curated by ChEMBL | Assay Description Binding affinity was measured against mutated human Opioid receptor delta 1 (W248L) | J Med Chem 41: 4767-76 (1998) Article DOI: 10.1021/jm980374r BindingDB Entry DOI: 10.7270/Q2DZ07FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50066659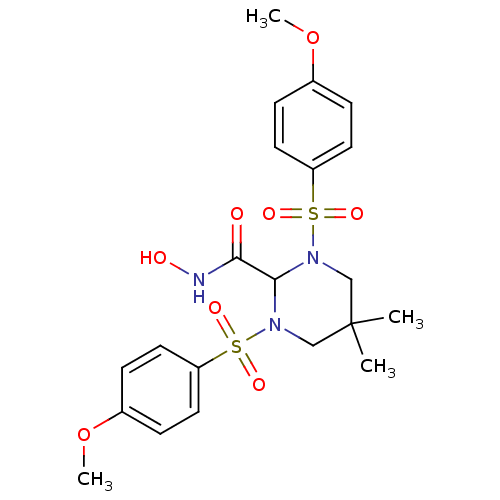 (1,3-BIS-(4-METHOXY-BENZENESULFONYL)-5,5-DIMETHYL-H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP9 catalytic domain incubated for 20 mins by fluorimetric assay | Bioorg Med Chem 20: 4164-71 (2012) Article DOI: 10.1016/j.bmc.2012.04.063 BindingDB Entry DOI: 10.7270/Q22808NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50571721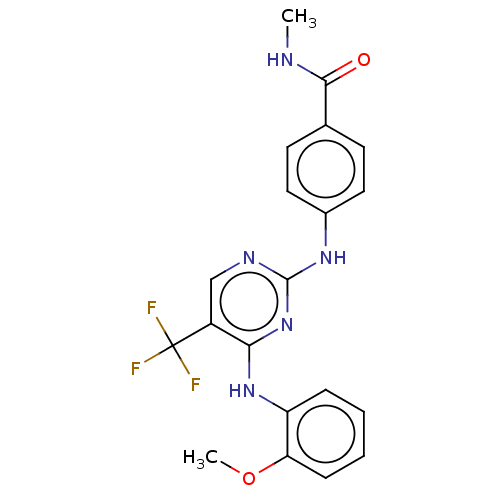 (CHEMBL4874046) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FAK (unknown origin) incubated for 40 mins by HTRF assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113573 BindingDB Entry DOI: 10.7270/Q2125XFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50610139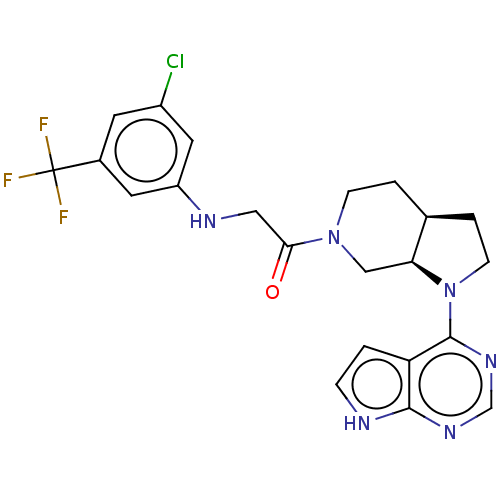 (CHEMBL5287698) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 783 total ) | Next | Last >> |