 Found 113 hits with Last Name = 'lightle' and Initial = 's'
Found 113 hits with Last Name = 'lightle' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50200120

(CHEMBL260091 | CHIR-090 | US10875832, Compound ChI...)Show SMILES C[C@@H](O)[C@H](NC(=O)c1ccc(cc1)C#Cc1ccc(CN2CCOCC2)cc1)C(=O)NO |r| Show InChI InChI=1S/C24H27N3O5/c1-17(28)22(24(30)26-31)25-23(29)21-10-8-19(9-11-21)3-2-18-4-6-20(7-5-18)16-27-12-14-32-15-13-27/h4-11,17,22,28,31H,12-16H2,1H3,(H,25,29)(H,26,30)/t17-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50478376

(BB-78485 | CHEMBL261713)Show SMILES ONC(=O)[C@@H](Cc1ccc2ccccc2c1)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C23H20N2O4S/c26-23(24-27)22(14-16-9-10-17-5-1-3-7-19(17)13-16)25-30(28,29)21-12-11-18-6-2-4-8-20(18)15-21/h1-13,15,22,25,27H,14H2,(H,24,26)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50074960

((R)-2-(3,4-Dimethoxy-5-propyl-phenyl)-4,5-dihydro-...)Show SMILES CCCc1cc(cc(OC)c1OC)C1=N[C@H](CO1)C(=O)NO |t:14| Show InChI InChI=1S/C15H20N2O5/c1-4-5-9-6-10(7-12(20-2)13(9)21-3)15-16-11(8-22-15)14(18)17-19/h6-7,11,19H,4-5,8H2,1-3H3,(H,17,18)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483375

(CHEMBL1236446)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](CC(=O)NO)CO[C@H](CO)[C@H]1O |r| Show InChI InChI=1S/C22H41NO7/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(26)30-22-17(14-19(25)23-28)16-29-18(15-24)21(22)27/h17-18,21-22,24,27-28H,2-16H2,1H3,(H,23,25)/t17-,18+,21+,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484911

(CHEMBL2012204)Show SMILES Cc1cc(no1)[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O5/c1-14-12-18(23-28-14)19(24)21(2,20(25)22-26)27-13-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-12,19,24,26H,13H2,1-2H3,(H,22,25)/t19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.376 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484910

(CHEMBL2012200)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccn[nH]1)C(=O)NO |r| Show InChI InChI=1S/C20H21N3O4/c1-20(19(25)23-26,18(24)17-11-12-21-22-17)27-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,24,26H,13H2,1H3,(H,21,22)(H,23,25)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484904

(CHEMBL2012203)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccon1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)21-25,18(23)17-11-12-27-22-17)26-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,23,25H,13H2,1H3,(H,21,24)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Escherichia coli) | BDBM50483411
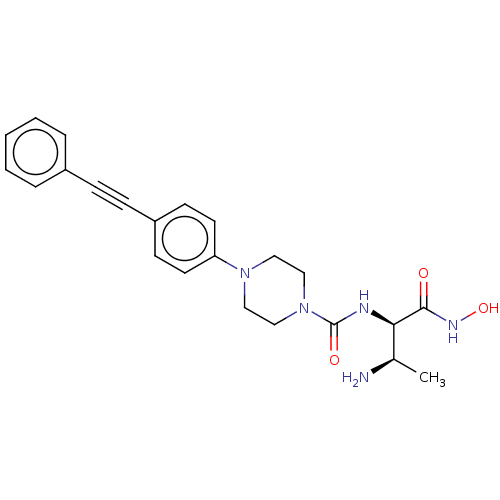
(CHEMBL1668464)Show SMILES C[C@@H](N)[C@@H](NC(=O)N1CCN(CC1)c1ccc(cc1)C#Cc1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H27N5O3/c1-17(24)21(22(29)26-31)25-23(30)28-15-13-27(14-16-28)20-11-9-19(10-12-20)8-7-18-5-3-2-4-6-18/h2-6,9-12,17,21,31H,13-16,24H2,1H3,(H,25,30)(H,26,29)/t17-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484896

(CHEMBL2012205)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1cc(CO)on1)C(=O)NO |r| Show InChI InChI=1S/C21H22N2O6/c1-21(20(26)22-27,19(25)18-11-17(12-24)29-23-18)28-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-11,19,24-25,27H,12-13H2,1H3,(H,22,26)/t19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484916

(CHEMBL2012199)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1c[nH]cn1)C(=O)NO |r| Show InChI InChI=1S/C20H21N3O4/c1-20(19(25)23-26,18(24)17-11-21-13-22-17)27-12-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-11,13,18,24,26H,12H2,1H3,(H,21,22)(H,23,25)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484895
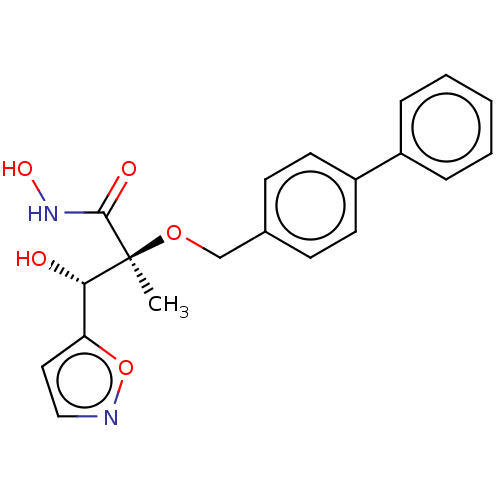
(CHEMBL2012202)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccno1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)22-25,18(23)17-11-12-21-27-17)26-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,18,23,25H,13H2,1H3,(H,22,24)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
Biotin carboxylase
(Escherichia coli (strain K12)) | BDBM3436
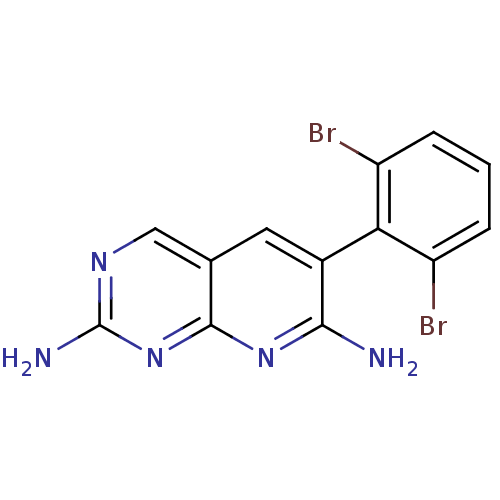
(6-(2,6-Dibromo-phenyl)-pyrido[2,3-d]pyrimidine-2,7...)Show SMILES Nc1ncc2cc(c(N)nc2n1)-c1c(Br)cccc1Br |(-5.18,.33,;-3.84,1.1,;-3.84,2.64,;-2.51,3.41,;-1.17,2.64,;.16,3.41,;1.49,2.64,;1.49,1.1,;2.83,.33,;.16,.33,;-1.17,1.1,;-2.51,.33,;2.83,3.41,;2.83,4.95,;1.49,5.72,;4.16,5.72,;5.49,4.95,;5.49,3.41,;4.16,2.64,;4.16,1.1,)| Show InChI InChI=1S/C13H9Br2N5/c14-8-2-1-3-9(15)10(8)7-4-6-5-18-13(17)20-12(6)19-11(7)16/h1-5H,(H4,16,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Pfizer
| Assay Description
Assays were performed in 384-well clear bottom plates (Corning; catalog no. 3702), that contained inhibitor solvated in DMSO. To each well of the pla... |
ACS Chem Biol 4: 473-83 (2009)
Article DOI: 10.1021/cb9000102
BindingDB Entry DOI: 10.7270/Q2WM1BR8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50491647
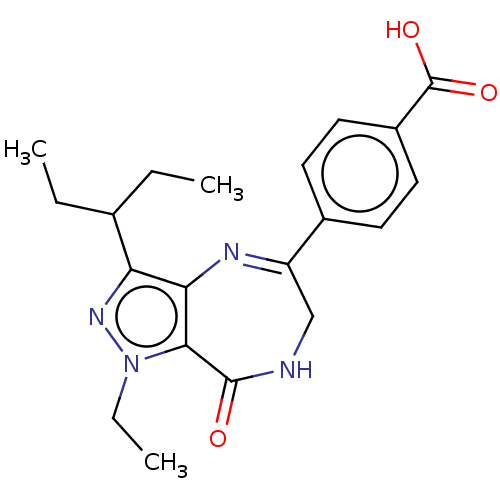
(CHEMBL2387019)Show SMILES CCC(CC)c1nn(CC)c2c1N=C(CNC2=O)c1ccc(cc1)C(O)=O |c:13| Show InChI InChI=1S/C20H24N4O3/c1-4-12(5-2)16-17-18(24(6-3)23-16)19(25)21-11-15(22-17)13-7-9-14(10-8-13)20(26)27/h7-10,12H,4-6,11H2,1-3H3,(H,21,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE4 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491689

(CHEMBL2387425)Show SMILES COc1ccc(cc1-c1cnc(nc1)N1CCCC1)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:22| Show InChI InChI=1S/C27H33N7O3/c1-27(2,3)24-22-23(34(32-24)11-12-35)25(36)28-16-20(31-22)17-7-8-21(37-4)19(13-17)18-14-29-26(30-15-18)33-9-5-6-10-33/h7-8,13-15,35H,5-6,9-12,16H2,1-4H3,(H,28,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491682
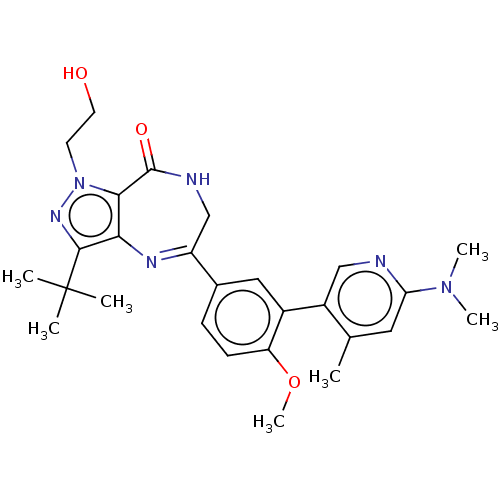
(CHEMBL2387423)Show SMILES COc1ccc(cc1-c1cnc(cc1C)N(C)C)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C27H34N6O3/c1-16-12-22(32(5)6)28-14-19(16)18-13-17(8-9-21(18)36-7)20-15-29-26(35)24-23(30-20)25(27(2,3)4)31-33(24)10-11-34/h8-9,12-14,34H,10-11,15H2,1-7H3,(H,29,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491698
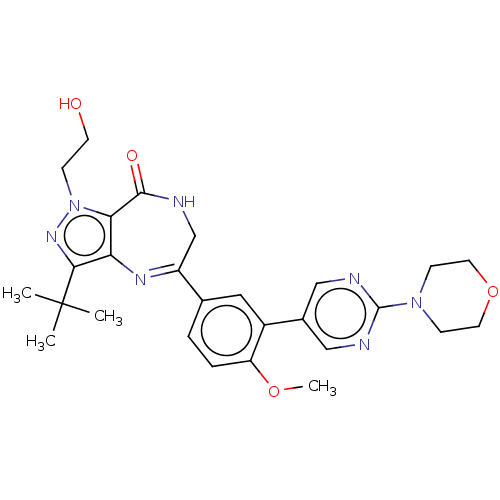
(CHEMBL2387426)Show SMILES COc1ccc(cc1-c1cnc(nc1)N1CCOCC1)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:23| Show InChI InChI=1S/C27H33N7O4/c1-27(2,3)24-22-23(34(32-24)7-10-35)25(36)28-16-20(31-22)17-5-6-21(37-4)19(13-17)18-14-29-26(30-15-18)33-8-11-38-12-9-33/h5-6,13-15,35H,7-12,16H2,1-4H3,(H,28,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491681
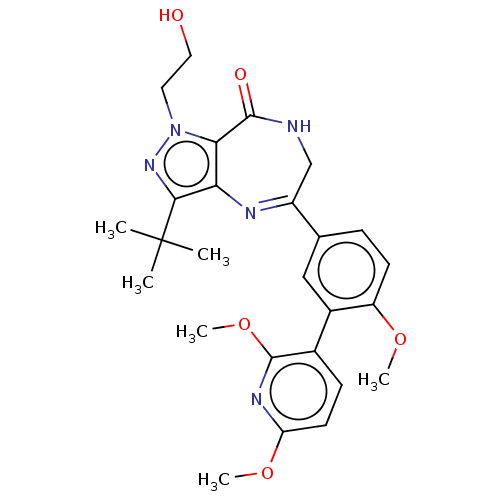
(CHEMBL2387427)Show SMILES COc1ccc(c(OC)n1)-c1cc(ccc1OC)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C26H31N5O5/c1-26(2,3)23-21-22(31(30-23)11-12-32)24(33)27-14-18(28-21)15-7-9-19(34-4)17(13-15)16-8-10-20(35-5)29-25(16)36-6/h7-10,13,32H,11-12,14H2,1-6H3,(H,27,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491680
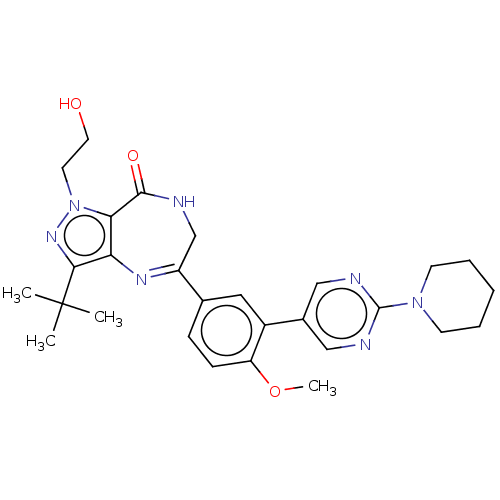
(CHEMBL2387429)Show SMILES COc1ccc(cc1-c1cnc(nc1)N1CCCCC1)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:23| Show InChI InChI=1S/C28H35N7O3/c1-28(2,3)25-23-24(35(33-25)12-13-36)26(37)29-17-21(32-23)18-8-9-22(38-4)20(14-18)19-15-30-27(31-16-19)34-10-6-5-7-11-34/h8-9,14-16,36H,5-7,10-13,17H2,1-4H3,(H,29,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491693
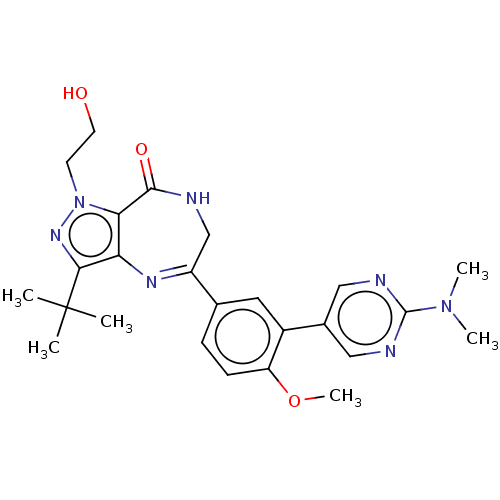
(CHEMBL2387428)Show SMILES COc1ccc(cc1-c1cnc(nc1)N(C)C)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:19| Show InChI InChI=1S/C25H31N7O3/c1-25(2,3)22-20-21(32(30-22)9-10-33)23(34)26-14-18(29-20)15-7-8-19(35-6)17(11-15)16-12-27-24(28-13-16)31(4)5/h7-8,11-13,33H,9-10,14H2,1-6H3,(H,26,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484894
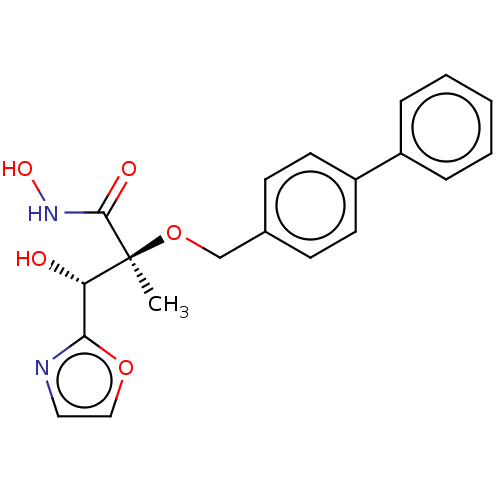
(CHEMBL2012201)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ncco1)C(=O)NO |r| Show InChI InChI=1S/C20H20N2O5/c1-20(19(24)22-25,17(23)18-21-11-12-26-18)27-13-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-12,17,23,25H,13H2,1H3,(H,22,24)/t17-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484917
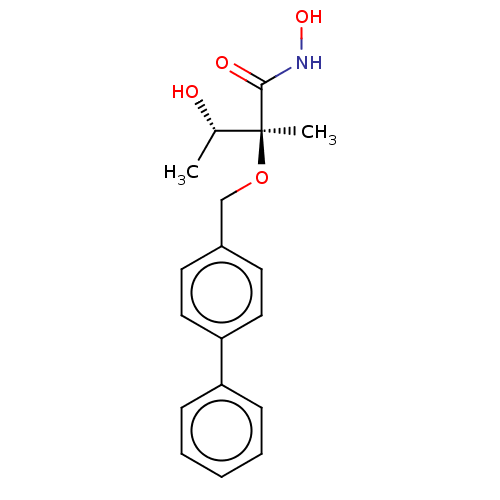
(CHEMBL2012187)Show SMILES C[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C18H21NO4/c1-13(20)18(2,17(21)19-22)23-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,20,22H,12H2,1-2H3,(H,19,21)/t13-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484899

(CHEMBL2012186)Show InChI InChI=1S/C18H21NO3/c1-18(13-20,17(21)19-22)12-11-14-7-9-16(10-8-14)15-5-3-2-4-6-15/h2-10,20,22H,11-13H2,1H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491679
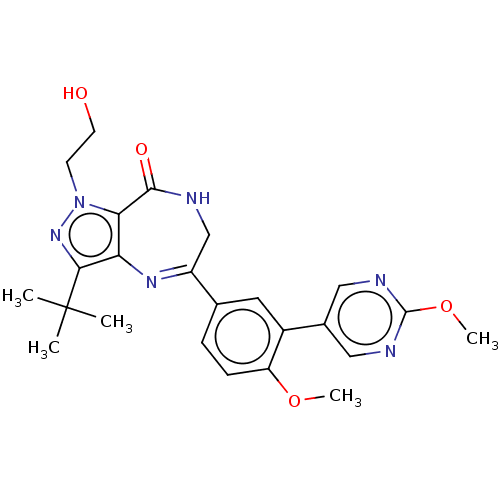
(CHEMBL2387430 | US11419874, Example 3)Show SMILES COc1ccc(cc1-c1cnc(OC)nc1)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:18| Show InChI InChI=1S/C24H28N6O4/c1-24(2,3)21-19-20(30(29-21)8-9-31)22(32)25-13-17(28-19)14-6-7-18(33-4)16(10-14)15-11-26-23(34-5)27-12-15/h6-7,10-12,31H,8-9,13H2,1-5H3,(H,25,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491694
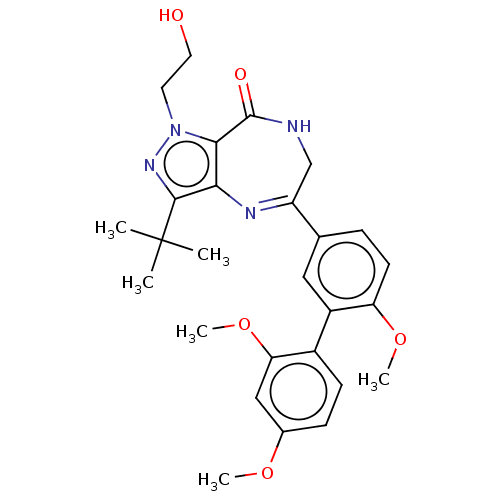
(CHEMBL2385104)Show SMILES COc1ccc(c(OC)c1)-c1cc(ccc1OC)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C27H32N4O5/c1-27(2,3)25-23-24(31(30-25)11-12-32)26(33)28-15-20(29-23)16-7-10-21(35-5)19(13-16)18-9-8-17(34-4)14-22(18)36-6/h7-10,13-14,32H,11-12,15H2,1-6H3,(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484900
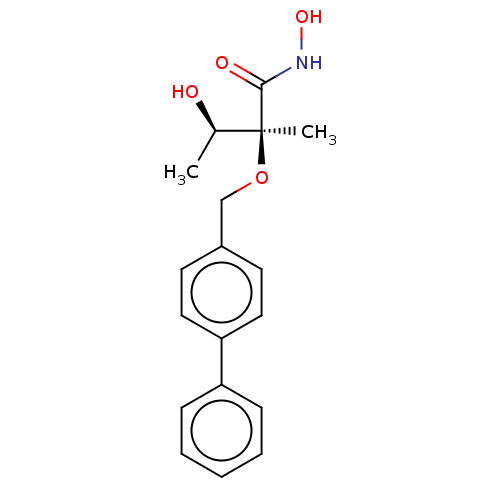
(CHEMBL2012188)Show SMILES C[C@@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C18H21NO4/c1-13(20)18(2,17(21)19-22)23-12-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-11,13,20,22H,12H2,1-2H3,(H,19,21)/t13-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491651
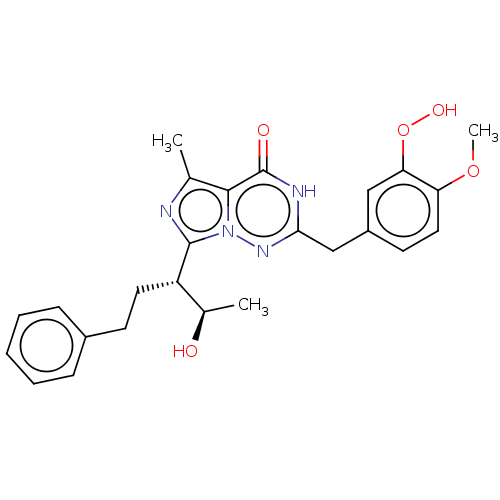
(CHEMBL2387143)Show SMILES COc1ccc(Cc2nn3c(nc(C)c3c(=O)[nH]2)[C@@H](CCc2ccccc2)[C@@H](C)O)cc1OO |r| Show InChI InChI=1S/C25H28N4O5/c1-15-23-25(31)27-22(14-18-10-12-20(33-3)21(13-18)34-32)28-29(23)24(26-15)19(16(2)30)11-9-17-7-5-4-6-8-17/h4-8,10,12-13,16,19,30,32H,9,11,14H2,1-3H3,(H,27,28,31)/t16-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491699
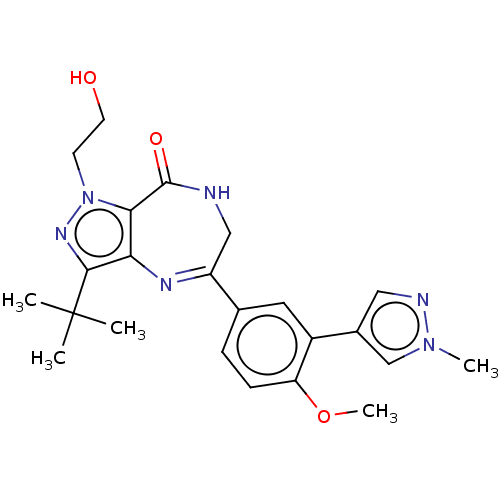
(CHEMBL2387422)Show SMILES COc1ccc(cc1-c1cnn(C)c1)C1=Nc2c(C(=O)NC1)n(CCO)nc2C(C)(C)C |t:16| Show InChI InChI=1S/C23H28N6O3/c1-23(2,3)21-19-20(29(27-21)8-9-30)22(31)24-12-17(26-19)14-6-7-18(32-5)16(10-14)15-11-25-28(4)13-15/h6-7,10-11,13,30H,8-9,12H2,1-5H3,(H,24,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491690
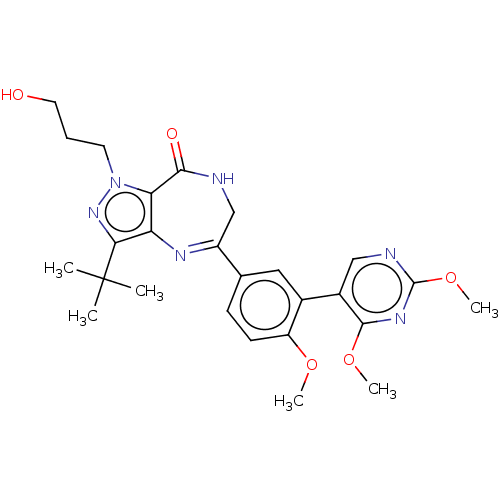
(CHEMBL2387441)Show SMILES COc1ccc(cc1-c1cnc(OC)nc1OC)C1=Nc2c(nn(CCCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C26H32N6O5/c1-26(2,3)22-20-21(32(31-22)10-7-11-33)23(34)27-14-18(29-20)15-8-9-19(35-4)16(12-15)17-13-28-25(37-6)30-24(17)36-5/h8-9,12-13,33H,7,10-11,14H2,1-6H3,(H,27,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
Biotin carboxylase
(Escherichia coli (strain K12)) | BDBM32642

(amino-oxazole, 2d)Show SMILES Cc1ccccc1CN(Cc1ccc2OCCOc2c1)C(=O)c1cnc(N)o1 Show InChI InChI=1S/C21H21N3O4/c1-14-4-2-3-5-16(14)13-24(20(25)19-11-23-21(22)28-19)12-15-6-7-17-18(10-15)27-9-8-26-17/h2-7,10-11H,8-9,12-13H2,1H3,(H2,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 8.0 | 23 |
Pfizer
| Assay Description
Assays were performed in 384-well clear bottom plates (Corning; catalog no. 3702), that contained inhibitor solvated in DMSO. To each well of the pla... |
ACS Chem Biol 4: 473-83 (2009)
Article DOI: 10.1021/cb9000102
BindingDB Entry DOI: 10.7270/Q2WM1BR8 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484907
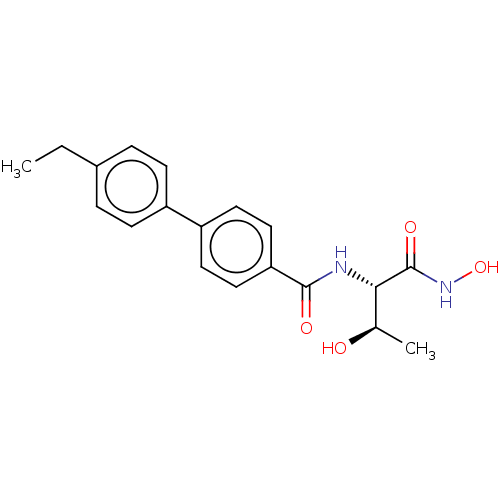
(CHEMBL2012182)Show SMILES CCc1ccc(cc1)-c1ccc(cc1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NO |r| Show InChI InChI=1S/C19H22N2O4/c1-3-13-4-6-14(7-5-13)15-8-10-16(11-9-15)18(23)20-17(12(2)22)19(24)21-25/h4-12,17,22,25H,3H2,1-2H3,(H,20,23)(H,21,24)/t12-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484901
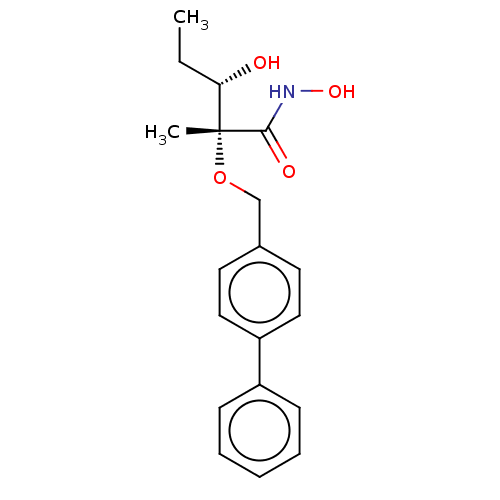
(CHEMBL2012189)Show SMILES CC[C@H](O)[C@@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C19H23NO4/c1-3-17(21)19(2,18(22)20-23)24-13-14-9-11-16(12-10-14)15-7-5-4-6-8-15/h4-12,17,21,23H,3,13H2,1-2H3,(H,20,22)/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491697
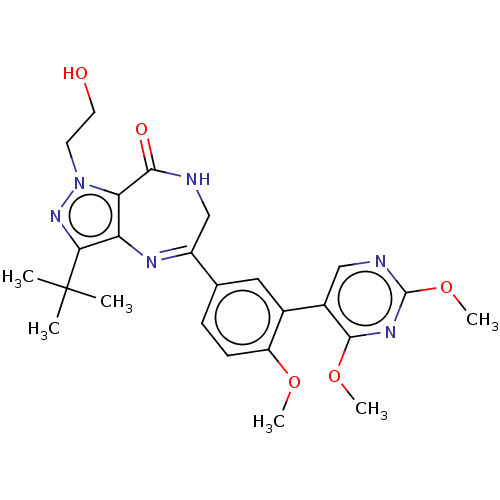
(CHEMBL2387424)Show SMILES COc1ccc(cc1-c1cnc(OC)nc1OC)C1=Nc2c(nn(CCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C25H30N6O5/c1-25(2,3)21-19-20(31(30-21)9-10-32)22(33)26-13-17(28-19)14-7-8-18(34-4)15(11-14)16-12-27-24(36-6)29-23(16)35-5/h7-8,11-12,32H,9-10,13H2,1-6H3,(H,26,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
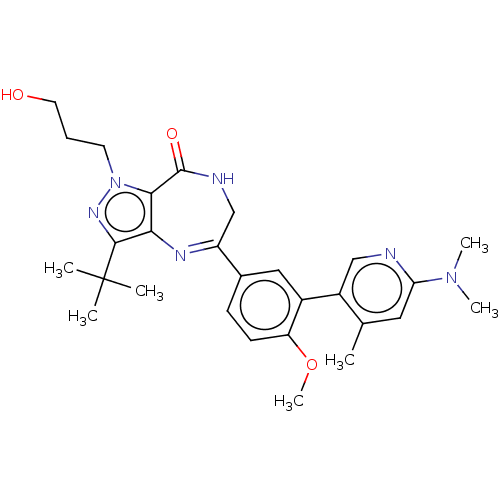
(Homo sapiens (Human)) | BDBM50491692
(CHEMBL2387436)Show SMILES COc1ccc(c(OC)c1)-c1cc(ccc1OC)C1=Nc2c(nn(CCCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C28H34N4O5/c1-28(2,3)26-24-25(32(31-26)12-7-13-33)27(34)29-16-21(30-24)17-8-11-22(36-5)20(14-17)19-10-9-18(35-4)15-23(19)37-6/h8-11,14-15,33H,7,12-13,16H2,1-6H3,(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484909
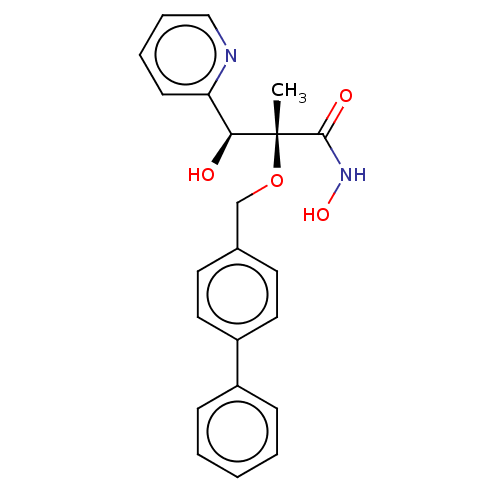
(CHEMBL2012196)Show SMILES C[C@@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccccn1)C(=O)NO |r| Show InChI InChI=1S/C22H22N2O4/c1-22(21(26)24-27,20(25)19-9-5-6-14-23-19)28-15-16-10-12-18(13-11-16)17-7-3-2-4-8-17/h2-14,20,25,27H,15H2,1H3,(H,24,26)/t20-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484906
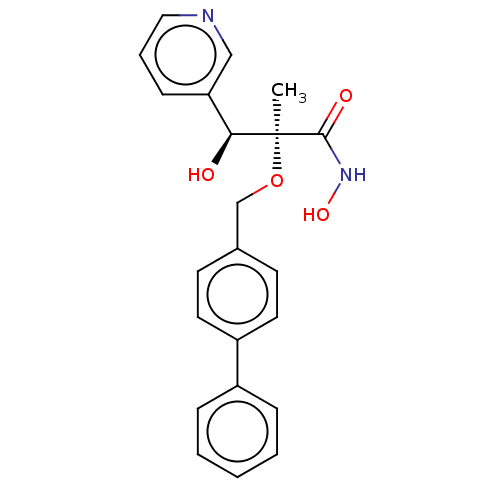
(CHEMBL2012195)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1cccnc1)C(=O)NO |r| Show InChI InChI=1S/C22H22N2O4/c1-22(21(26)24-27,20(25)19-8-5-13-23-14-19)28-15-16-9-11-18(12-10-16)17-6-3-2-4-7-17/h2-14,20,25,27H,15H2,1H3,(H,24,26)/t20-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491683
(CHEMBL2387440)Show SMILES COc1ccc(cc1-c1cnc(cc1C)N(C)C)C1=Nc2c(nn(CCCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C28H36N6O3/c1-17-13-23(33(5)6)29-15-20(17)19-14-18(9-10-22(19)37-7)21-16-30-27(36)25-24(31-21)26(28(2,3)4)32-34(25)11-8-12-35/h9-10,13-15,35H,8,11-12,16H2,1-7H3,(H,30,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484913
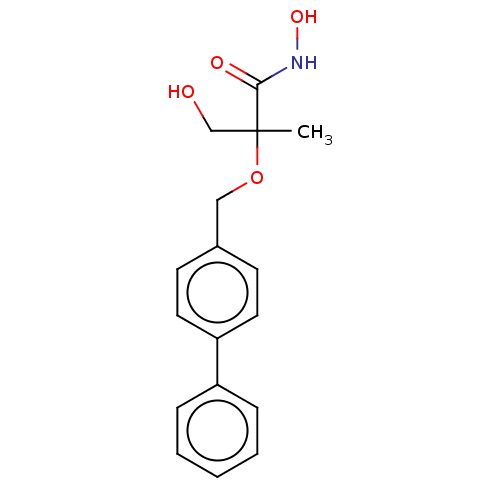
(CHEMBL2012185)Show InChI InChI=1S/C17H19NO4/c1-17(12-19,16(20)18-21)22-11-13-7-9-15(10-8-13)14-5-3-2-4-6-14/h2-10,19,21H,11-12H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491645
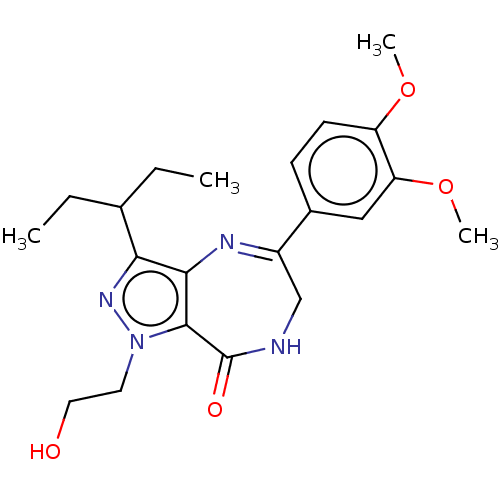
(CHEMBL2387135)Show SMILES CCC(CC)c1nn(CCO)c2c1N=C(CNC2=O)c1ccc(OC)c(OC)c1 |c:14| Show InChI InChI=1S/C21H28N4O4/c1-5-13(6-2)18-19-20(25(24-18)9-10-26)21(27)22-12-15(23-19)14-7-8-16(28-3)17(11-14)29-4/h7-8,11,13,26H,5-6,9-10,12H2,1-4H3,(H,22,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484915
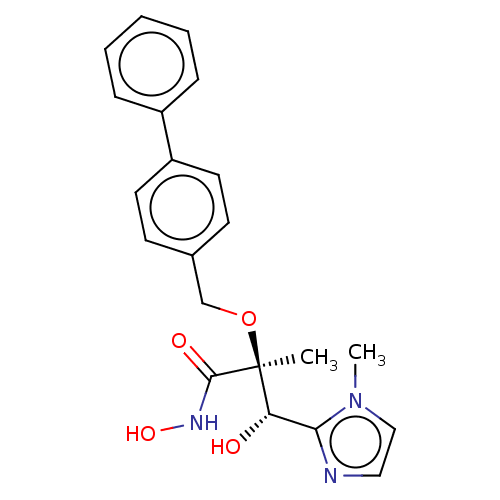
(CHEMBL2012198)Show SMILES Cn1ccnc1[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H23N3O4/c1-21(20(26)23-27,18(25)19-22-12-13-24(19)2)28-14-15-8-10-17(11-9-15)16-6-4-3-5-7-16/h3-13,18,25,27H,14H2,1-2H3,(H,23,26)/t18-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484893
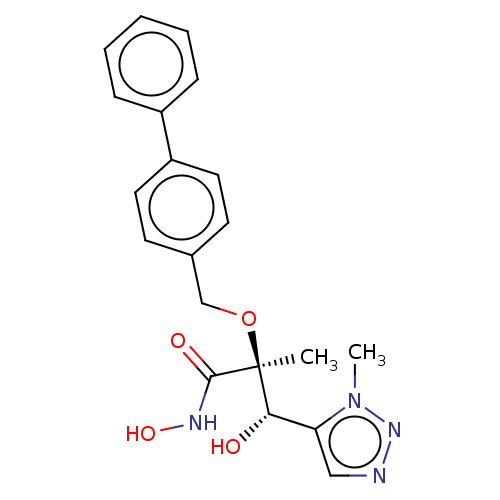
(CHEMBL2012197)Show SMILES Cn1nncc1[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C20H22N4O4/c1-20(19(26)22-27,18(25)17-12-21-23-24(17)2)28-13-14-8-10-16(11-9-14)15-6-4-3-5-7-15/h3-12,18,25,27H,13H2,1-2H3,(H,22,26)/t18-,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484914
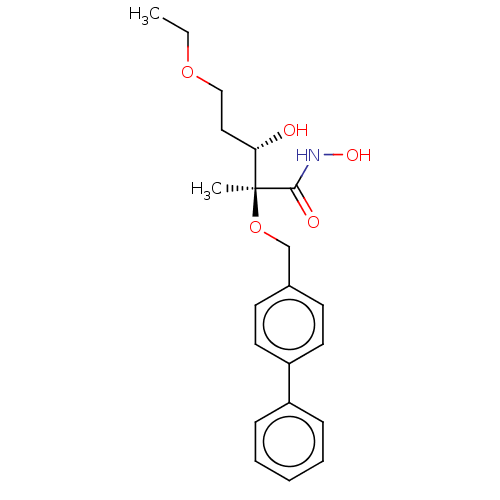
(CHEMBL2012193)Show SMILES CCOCC[C@H](O)[C@](C)(OCc1ccc(cc1)-c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C21H27NO5/c1-3-26-14-13-19(23)21(2,20(24)22-25)27-15-16-9-11-18(12-10-16)17-7-5-4-6-8-17/h4-12,19,23,25H,3,13-15H2,1-2H3,(H,22,24)/t19-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50491650
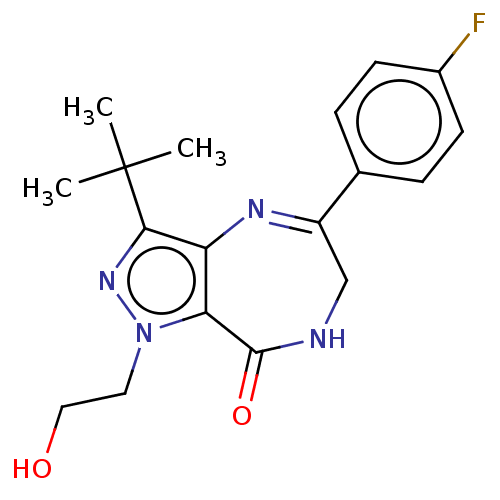
(CHEMBL2387020)Show SMILES CC(C)(C)c1nn(CCO)c2c1N=C(CNC2=O)c1ccc(F)cc1 |c:13| Show InChI InChI=1S/C18H21FN4O2/c1-18(2,3)16-14-15(23(22-16)8-9-24)17(25)20-10-13(21-14)11-4-6-12(19)7-5-11/h4-7,24H,8-10H2,1-3H3,(H,20,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE4 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491684
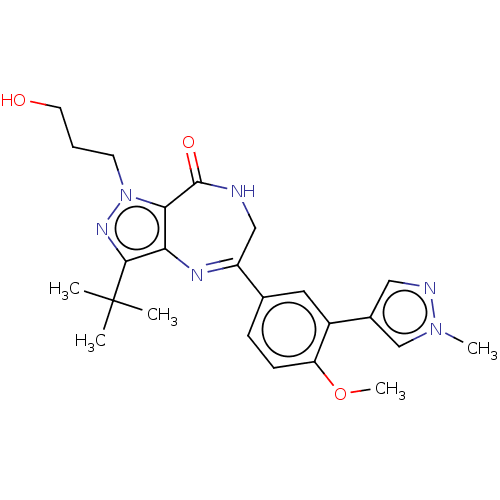
(CHEMBL2387439)Show SMILES COc1ccc(cc1-c1cnn(C)c1)C1=Nc2c(C(=O)NC1)n(CCCO)nc2C(C)(C)C |t:16| Show InChI InChI=1S/C24H30N6O3/c1-24(2,3)22-20-21(30(28-22)9-6-10-31)23(32)25-13-18(27-20)15-7-8-19(33-5)17(11-15)16-12-26-29(4)14-16/h7-8,11-12,14,31H,6,9-10,13H2,1-5H3,(H,25,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491695
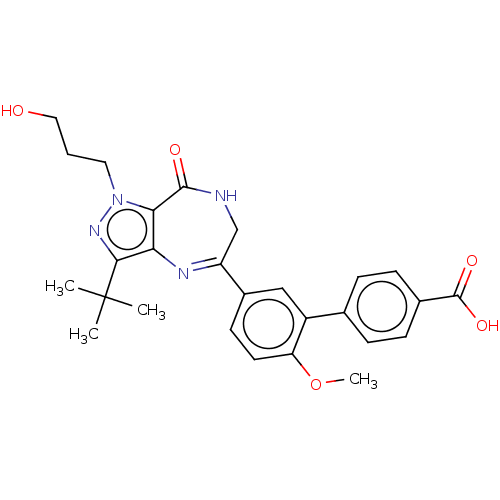
(CHEMBL2387437)Show SMILES COc1ccc(cc1-c1ccc(cc1)C(O)=O)C1=Nc2c(nn(CCCO)c2C(=O)NC1)C(C)(C)C |t:19| Show InChI InChI=1S/C27H30N4O5/c1-27(2,3)24-22-23(31(30-24)12-5-13-32)25(33)28-15-20(29-22)18-10-11-21(36-4)19(14-18)16-6-8-17(9-7-16)26(34)35/h6-11,14,32H,5,12-13,15H2,1-4H3,(H,28,33)(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491644

(CHEMBL2387139)Show SMILES CCC(CC)c1nn(CCCO)c2c1N=C(CNC2=O)c1ccccc1 |c:15| Show InChI InChI=1S/C20H26N4O2/c1-3-14(4-2)17-18-19(24(23-17)11-8-12-25)20(26)21-13-16(22-18)15-9-6-5-7-10-15/h5-7,9-10,14,25H,3-4,8,11-13H2,1-2H3,(H,21,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491643
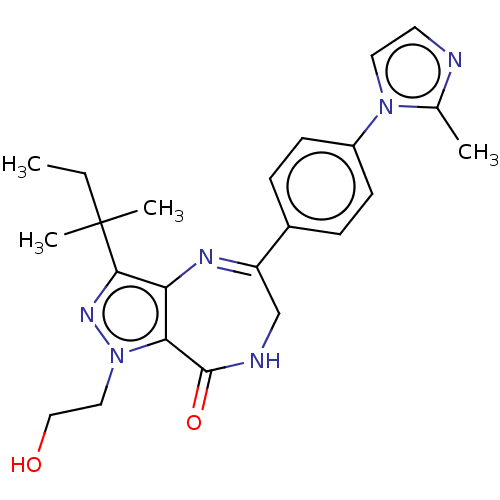
(CHEMBL2387140)Show SMILES CCC(C)(C)c1nn(CCO)c2c1N=C(CNC2=O)c1ccc(cc1)-n1ccnc1C |c:14| Show InChI InChI=1S/C23H28N6O2/c1-5-23(3,4)21-19-20(29(27-21)12-13-30)22(31)25-14-18(26-19)16-6-8-17(9-7-16)28-11-10-24-15(28)2/h6-11,30H,5,12-14H2,1-4H3,(H,25,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491643

(CHEMBL2387140)Show SMILES CCC(C)(C)c1nn(CCO)c2c1N=C(CNC2=O)c1ccc(cc1)-n1ccnc1C |c:14| Show InChI InChI=1S/C23H28N6O2/c1-5-23(3,4)21-19-20(29(27-21)12-13-30)22(31)25-14-18(26-19)16-6-8-17(9-7-16)28-11-10-24-15(28)2/h6-11,30H,5,12-14H2,1-4H3,(H,25,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50491685
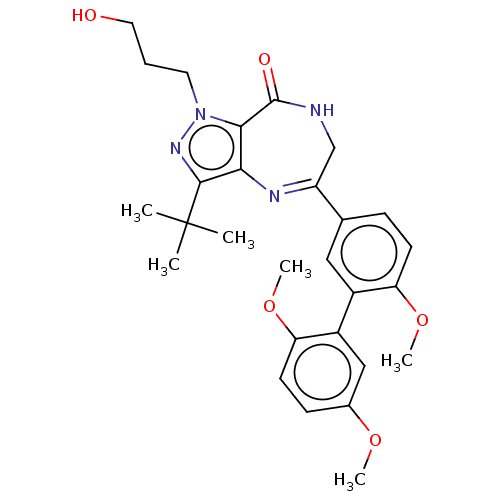
(CHEMBL2387435)Show SMILES COc1ccc(OC)c(c1)-c1cc(ccc1OC)C1=Nc2c(nn(CCCO)c2C(=O)NC1)C(C)(C)C |t:20| Show InChI InChI=1S/C28H34N4O5/c1-28(2,3)26-24-25(32(31-26)12-7-13-33)27(34)29-16-21(30-24)17-8-10-22(36-5)19(14-17)20-15-18(35-4)9-11-23(20)37-6/h8-11,14-15,33H,7,12-13,16H2,1-6H3,(H,29,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3443-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.082
BindingDB Entry DOI: 10.7270/Q2C53PR1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM50491645

(CHEMBL2387135)Show SMILES CCC(CC)c1nn(CCO)c2c1N=C(CNC2=O)c1ccc(OC)c(OC)c1 |c:14| Show InChI InChI=1S/C21H28N4O4/c1-5-13(6-2)18-19-20(25(24-18)9-10-26)21(27)22-12-15(23-19)14-7-8-16(28-3)17(11-14)29-4/h7-8,11,13,26H,5-6,9-10,12H2,1-4H3,(H,22,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE4 (unknown origin) |
Bioorg Med Chem Lett 23: 3438-42 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.072
BindingDB Entry DOI: 10.7270/Q2MP5665 |
More data for this
Ligand-Target Pair | |
UDP-3-O-acyl-N-acetylglucosamine deacetylase
(Pseudomonas aeruginosa) | BDBM50484903
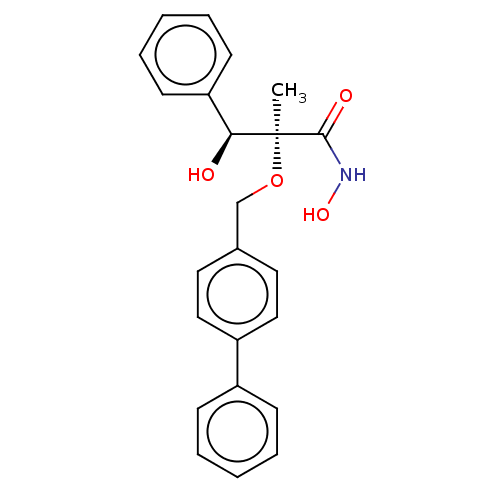
(CHEMBL2012194)Show SMILES C[C@](OCc1ccc(cc1)-c1ccccc1)([C@@H](O)c1ccccc1)C(=O)NO |r| Show InChI InChI=1S/C23H23NO4/c1-23(22(26)24-27,21(25)20-10-6-3-7-11-20)28-16-17-12-14-19(15-13-17)18-8-4-2-5-9-18/h2-15,21,25,27H,16H2,1H3,(H,24,26)/t21-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LpxC |
Bioorg Med Chem Lett 22: 2536-43 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.140
BindingDB Entry DOI: 10.7270/Q2JS9T9W |
More data for this
Ligand-Target Pair | |


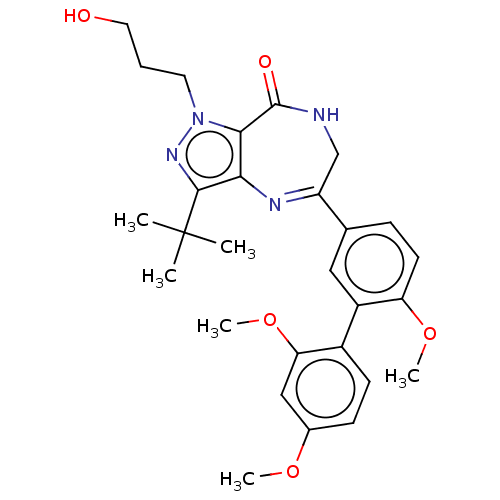
 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data