 Found 13 hits with Last Name = 'lim' and Initial = 'ss'
Found 13 hits with Last Name = 'lim' and Initial = 'ss' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aldehyde dehydrogenase, cytosolic 1
(Rattus norvegicus) | BDBM50292380
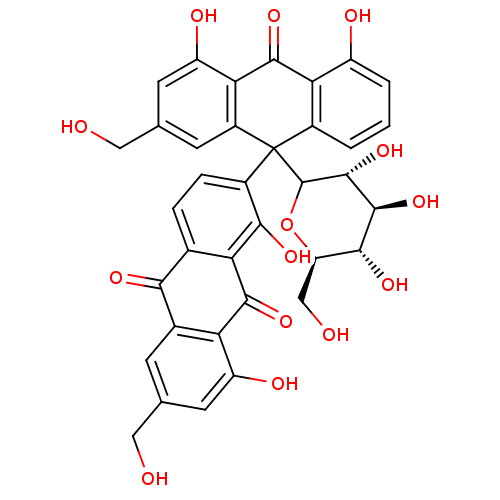
(10'-D-glucopyranosyl-1,8,1',8'-tetrahydroxy-3,3'-b...)Show SMILES OC[C@H]1OC([C@H](O)[C@@H](O)[C@@H]1O)C1(c2ccc3C(=O)c4cc(CO)cc(O)c4C(=O)c3c2O)c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C36H30O14/c37-10-13-6-16-24(21(41)8-13)31(46)25-15(28(16)43)4-5-18(29(25)44)36(35-34(49)33(48)30(45)23(12-39)50-35)17-2-1-3-20(40)26(17)32(47)27-19(36)7-14(11-38)9-22(27)42/h1-9,23,30,33-35,37-42,44-45,48-49H,10-12H2/t23-,30-,33+,34-,35?,36?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Rattus norvegicus) | BDBM50292380

(10'-D-glucopyranosyl-1,8,1',8'-tetrahydroxy-3,3'-b...)Show SMILES OC[C@H]1OC([C@H](O)[C@@H](O)[C@@H]1O)C1(c2ccc3C(=O)c4cc(CO)cc(O)c4C(=O)c3c2O)c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C36H30O14/c37-10-13-6-16-24(21(41)8-13)31(46)25-15(28(16)43)4-5-18(29(25)44)36(35-34(49)33(48)30(45)23(12-39)50-35)17-2-1-3-20(40)26(17)32(47)27-19(36)7-14(11-38)9-22(27)42/h1-9,23,30,33-35,37-42,44-45,48-49H,10-12H2/t23-,30-,33+,34-,35?,36?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat mitochondrial aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, cytosolic 1
(Rattus norvegicus) | BDBM50292380

(10'-D-glucopyranosyl-1,8,1',8'-tetrahydroxy-3,3'-b...)Show SMILES OC[C@H]1OC([C@H](O)[C@@H](O)[C@@H]1O)C1(c2ccc3C(=O)c4cc(CO)cc(O)c4C(=O)c3c2O)c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C36H30O14/c37-10-13-6-16-24(21(41)8-13)31(46)25-15(28(16)43)4-5-18(29(25)44)36(35-34(49)33(48)30(45)23(12-39)50-35)17-2-1-3-20(40)26(17)32(47)27-19(36)7-14(11-38)9-22(27)42/h1-9,23,30,33-35,37-42,44-45,48-49H,10-12H2/t23-,30-,33+,34-,35?,36?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Rattus norvegicus) | BDBM50292380

(10'-D-glucopyranosyl-1,8,1',8'-tetrahydroxy-3,3'-b...)Show SMILES OC[C@H]1OC([C@H](O)[C@@H](O)[C@@H]1O)C1(c2ccc3C(=O)c4cc(CO)cc(O)c4C(=O)c3c2O)c2cccc(O)c2C(=O)c2c(O)cc(CO)cc12 |r| Show InChI InChI=1S/C36H30O14/c37-10-13-6-16-24(21(41)8-13)31(46)25-15(28(16)43)4-5-18(29(25)44)36(35-34(49)33(48)30(45)23(12-39)50-35)17-2-1-3-20(40)26(17)32(47)27-19(36)7-14(11-38)9-22(27)42/h1-9,23,30,33-35,37-42,44-45,48-49H,10-12H2/t23-,30-,33+,34-,35?,36?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat mitochondrial aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50308654
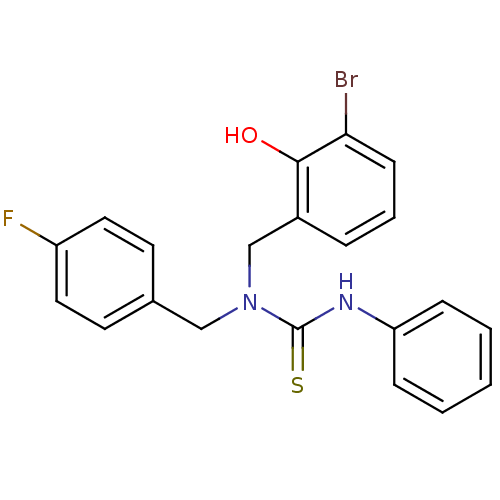
(1-(5-Bromo-2-hydroxybenzyl)-1-(4-fluorobenzyl)-3-p...)Show SMILES Oc1c(Br)cccc1CN(Cc1ccc(F)cc1)C(=S)Nc1ccccc1 Show InChI InChI=1S/C21H18BrFN2OS/c22-19-8-4-5-16(20(19)26)14-25(13-15-9-11-17(23)12-10-15)21(27)24-18-6-2-1-3-7-18/h1-12,26H,13-14H2,(H,24,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HER2 (unknown origin) by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127658
BindingDB Entry DOI: 10.7270/Q2X3524J |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Candida albicans (strain SC5314 / ATCC MYA-2876) (...) | BDBM50590139
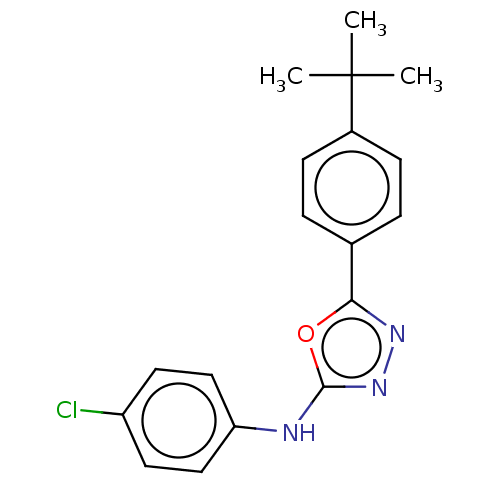
(CHEMBL5196919) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00196a
BindingDB Entry DOI: 10.7270/Q2K64P16 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50308637
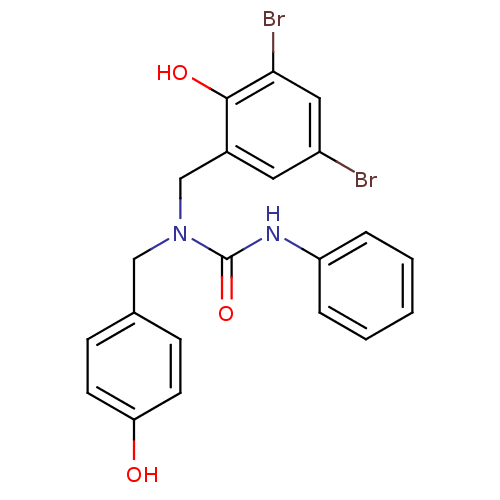
(1-(3,5-Dibromo-2-hydroxybenzyl)-1-(4-hydroxybenzyl...)Show SMILES Oc1ccc(CN(Cc2cc(Br)cc(Br)c2O)C(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C21H18Br2N2O3/c22-16-10-15(20(27)19(23)11-16)13-25(12-14-6-8-18(26)9-7-14)21(28)24-17-4-2-1-3-5-17/h1-11,26-27H,12-13H2,(H,24,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HER2 (unknown origin) by ELISA |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127658
BindingDB Entry DOI: 10.7270/Q2X3524J |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, mitochondrial
(Rattus norvegicus) | BDBM50172756
(Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...)Show InChI InChI=1S/C11H13N/c1-3-9-12(2)10-11-7-5-4-6-8-11/h1,4-8H,9-10H2,2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat mitochondrial aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, cytosolic 1
(Rattus norvegicus) | BDBM50085551
(1,8-Dihydroxy-3-hydroxymethyl-anthraquinone | 1,8-...)Show InChI InChI=1S/C15H10O5/c16-6-7-4-9-13(11(18)5-7)15(20)12-8(14(9)19)2-1-3-10(12)17/h1-5,16-18H,6H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, cytosolic 1
(Rattus norvegicus) | BDBM50292381
(CHEMBL518845 | aloenin)Show SMILES COc1cc(oc(=O)c1)-c1c(C)cc(O)cc1O[C@@H]1O[C@@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C19H22O10/c1-8-3-9(21)4-11(15(8)12-5-10(26-2)6-14(22)27-12)28-19-18(25)17(24)16(23)13(7-20)29-19/h3-6,13,16-21,23-25H,7H2,1-2H3/t13-,16+,17-,18+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Lanosterol 14-alpha demethylase
(Candida albicans (strain SC5314 / ATCC MYA-2876) (...) | BDBM25817
(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00196a
BindingDB Entry DOI: 10.7270/Q2K64P16 |
More data for this
Ligand-Target Pair | |
Aldehyde dehydrogenase, cytosolic 1
(Rattus norvegicus) | BDBM50172756

(Benzyl-methyl-prop-2-ynyl-amine | CHEMBL673 | Euto...)Show InChI InChI=1S/C11H13N/c1-3-9-12(2)10-11-7-5-4-6-8-11/h1,4-8H,9-10H2,2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Sprague-Dawley rat cytosolic aldehyde dehydrogenase |
J Nat Prod 60: 1180-1182 (1997)
Article DOI: 10.1021/np9703104
BindingDB Entry DOI: 10.7270/Q2DR2VHR |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50590139

(CHEMBL5196919) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.05E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1039/d2md00196a
BindingDB Entry DOI: 10.7270/Q2K64P16 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data