

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human neurotensin receptor 1 | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615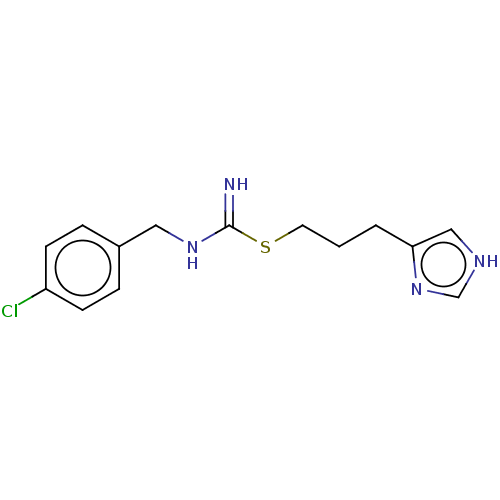 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of clobenpropit-BODIPY-630/650 from recombinant human NLuc-fused H3R expressed in HEK293T cells by Nano-BRET assay | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H3R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes co... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509512 (CHEMBL4579623) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human neurotensin receptor 1 | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50538682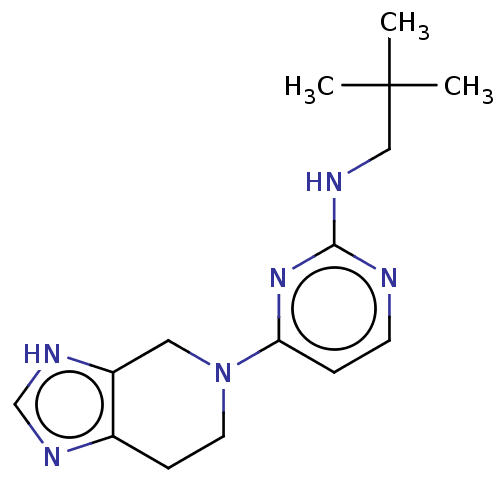 (CHEMBL4597218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H3R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509515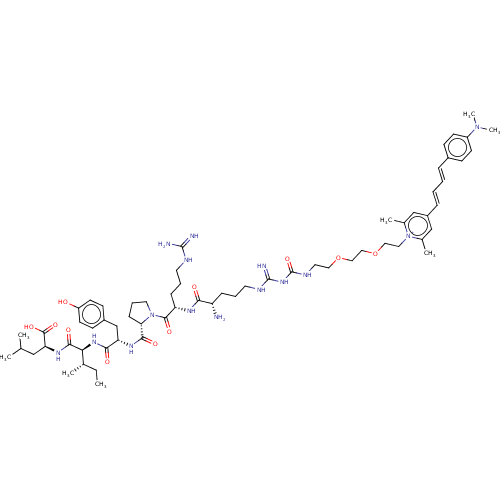 (CHEMBL4463399) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50538678 (CHEMBL4642520) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50509508 (CHEMBL4534768) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50538682 (CHEMBL4597218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H4R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM50538682 (CHEMBL4597218) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to mouse recombinant NLuc/GPCR-fused H4R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human neurotensin receptor 1 | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50509510 (CHEMBL4564746) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509511 (CHEMBL4457798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509515 (CHEMBL4463399) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509511 (CHEMBL4457798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509513 (CHEMBL4537266) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509513 (CHEMBL4537266) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509508 (CHEMBL4534768) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509514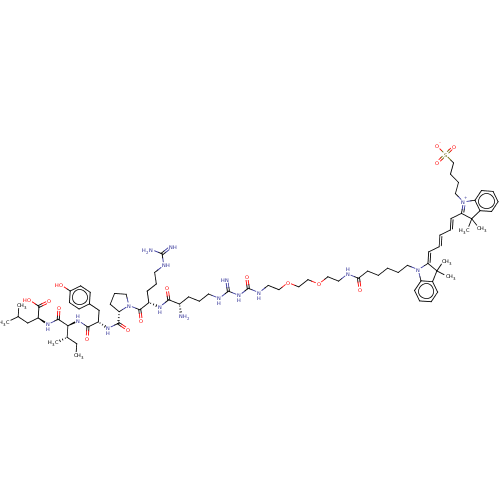 (CHEMBL4584647) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50538677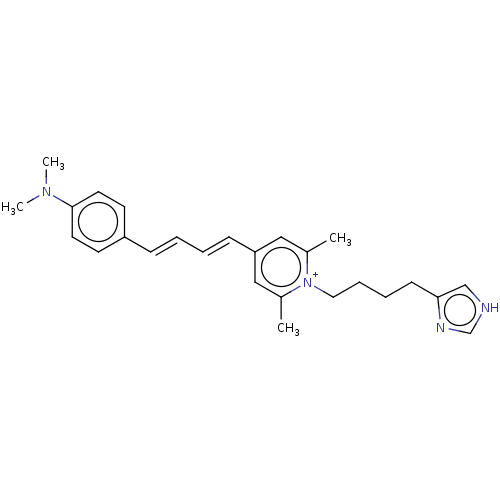 (CHEMBL4635634) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50214615 (CHEBI:64177 | Clobenpropit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant H3R expressed in human SK-N-MC cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509508 (CHEMBL4534768) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509516 (CHEMBL4592541) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50538682 (CHEMBL4597218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect ce... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50509072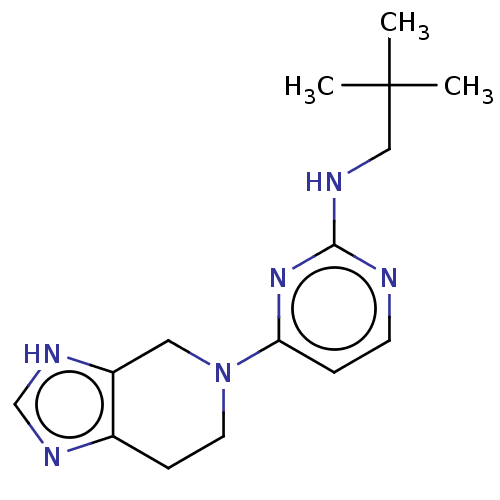 (CHEMBL4454158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 3 nM [3H]Nalpha-methylhistamine from human H3R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition bind... | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50509513 (CHEMBL4537266) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509509 (CHEMBL4566971) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509078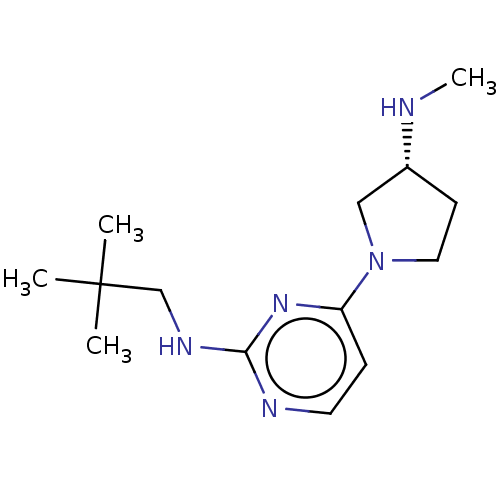 (CHEMBL4443126) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 40 nM [3H]-histamine from human H4R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50509511 (CHEMBL4457798) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509510 (CHEMBL4564746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H4R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50170164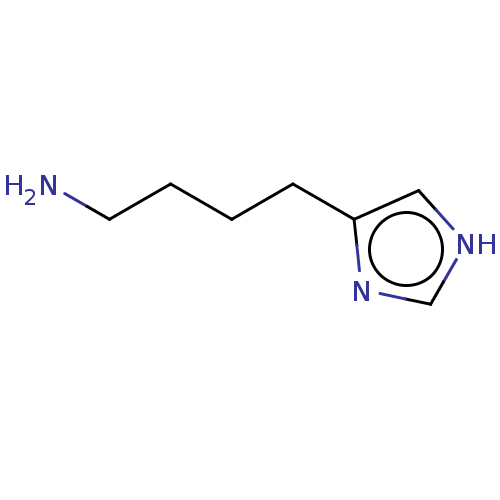 (Imbutamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect ce... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509514 (CHEMBL4584647) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50538682 (CHEMBL4597218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-histamine from Galphai2/Gbeta1gamma2-coupled human recombinant H4R expressed in baculovirus infected Sf9 insect cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509072 (CHEMBL4454158) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 10 nM [3H]-histamine from human H4R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50509510 (CHEMBL4564746) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 stably expressed in CHO cells incubated in dark after 30 mins by liquid scintillation... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 2 (Homo sapiens (Human)) | BDBM50509516 (CHEMBL4592541) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 2 in HEK293 cells homogenate incubated in dark after 30 mins by liquid scintillation co... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509071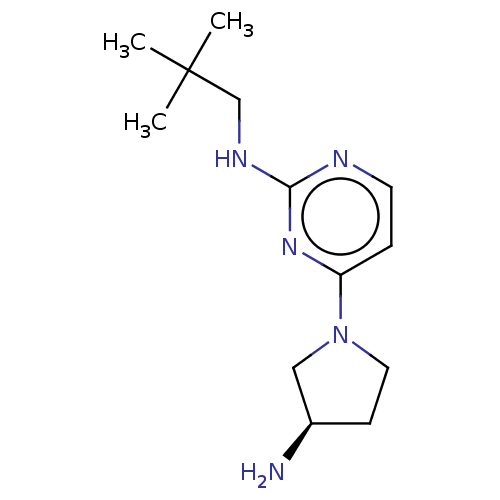 (CHEMBL4455324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 10 nM [3H]-histamine from human H4R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50509081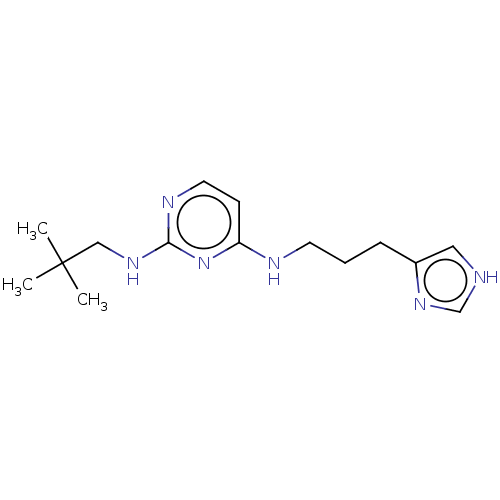 (CHEMBL4470527) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 2 nM [3H]UR-PI294 from human H3R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50509077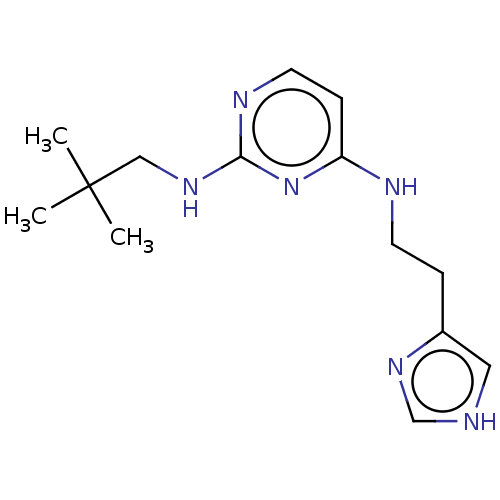 (CHEMBL4473753) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 2 nM [3H]UR-PI294 from human H3R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes co... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human recombinant H3R expressed in human SK-N-MC cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50538681 (CHEMBL4595327) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to human recombinant NLuc/GPCR-fused H4R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Mus musculus (mouse)) | BDBM22566 (5-chloro-2-[(4-methylpiperazin-1-yl)carbonyl]-1H-i...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Inhibition of UR-DEBa242 binding to mouse recombinant NLuc/GPCR-fused H4R expressed in HEK293T cells measured after 30 mins by furimazine substrate b... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50538676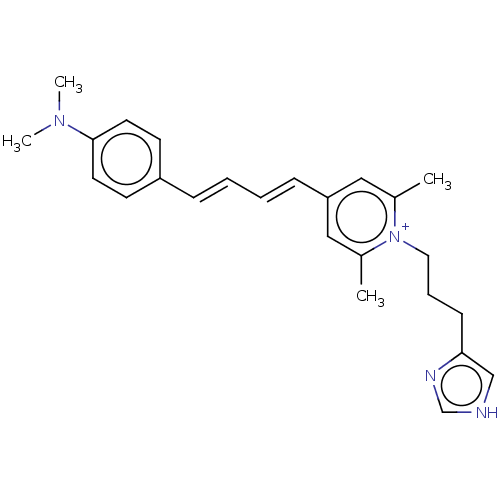 (CHEMBL4636487) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-PI294 from Galphai2/Gbeta1gamma2-coupled human recombinant H3R expressed in baculovirus infected Sf9 insect cell membranes | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50509065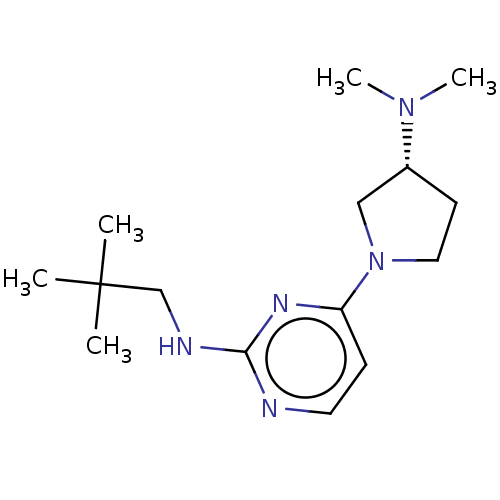 (CHEMBL4439142) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 10 nM [3H]-histamine from human H4R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 BindingDB Entry DOI: 10.7270/Q28K7DCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50121205 (CHEBI:18295 | Histamine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-histamine from Galphai2/Gbeta1gamma2-coupled human recombinant H4R expressed in baculovirus infected Sf9 insect cell membranes m... | J Med Chem 63: 5297-5311 (2020) Article DOI: 10.1021/acs.jmedchem.0c00160 BindingDB Entry DOI: 10.7270/Q2WM1HZ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 479 total ) | Next | Last >> |