 Found 192 hits with Last Name = 'liu' and Initial = 'zp'
Found 192 hits with Last Name = 'liu' and Initial = 'zp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497089
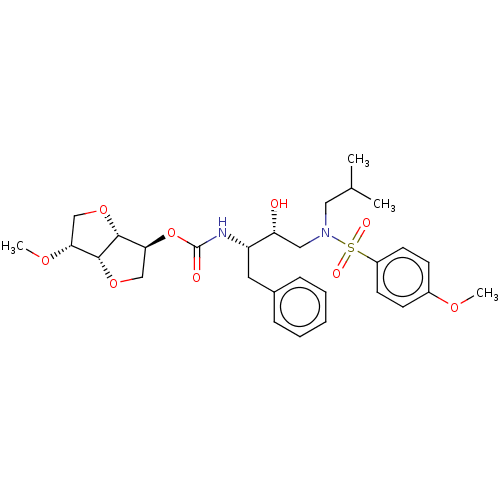
(CHEMBL3263091)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(OC)cc3)[C@@]1([H])OC[C@H]2OC |r| Show InChI InChI=1S/C29H40N2O9S/c1-19(2)15-31(41(34,35)22-12-10-21(36-3)11-13-22)16-24(32)23(14-20-8-6-5-7-9-20)30-29(33)40-26-18-39-27-25(37-4)17-38-28(26)27/h5-13,19,23-28,32H,14-18H2,1-4H3,(H,30,33)/t23-,24+,25+,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497086
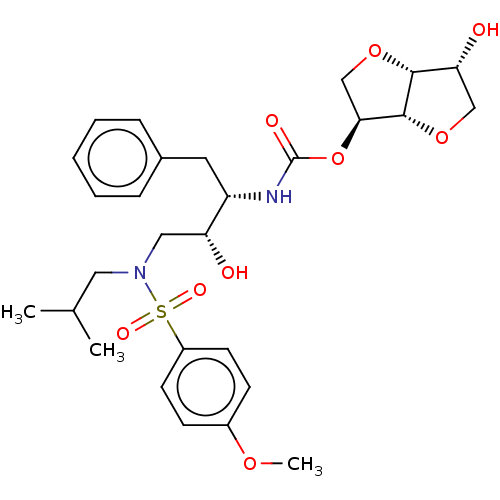
(CHEMBL3263090)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(OC)cc3)[C@@]1([H])OC[C@H]2O |r| Show InChI InChI=1S/C28H38N2O9S/c1-18(2)14-30(40(34,35)21-11-9-20(36-3)10-12-21)15-23(31)22(13-19-7-5-4-6-8-19)29-28(33)39-25-17-38-26-24(32)16-37-27(25)26/h4-12,18,22-27,31-32H,13-17H2,1-3H3,(H,29,33)/t22-,23+,24+,25-,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497088

(CHEMBL3263089)Show SMILES [H][C@]12OC[C@@H](O[N+]([O-])=O)[C@@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r| Show InChI InChI=1S/C28H37N3O11S/c1-18(2)14-30(43(36,37)21-11-9-20(38-3)10-12-21)15-23(32)22(13-19-7-5-4-6-8-19)29-28(33)41-24-16-39-27-25(42-31(34)35)17-40-26(24)27/h4-12,18,22-27,32H,13-17H2,1-3H3,(H,29,33)/t22-,23+,24-,25+,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497090
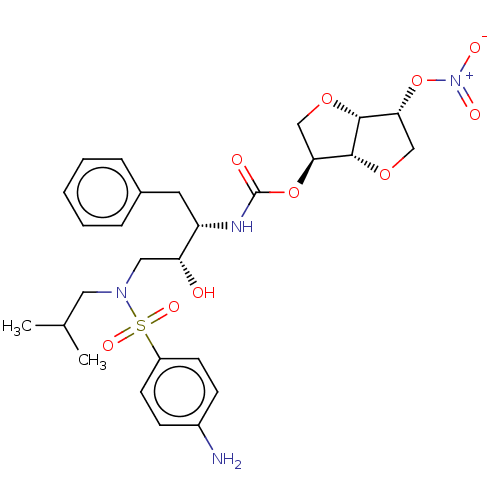
(CHEMBL3263092)Show SMILES [H][C@]12OC[C@@H](O[N+]([O-])=O)[C@@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H36N4O10S/c1-17(2)13-30(42(36,37)20-10-8-19(28)9-11-20)14-22(32)21(12-18-6-4-3-5-7-18)29-27(33)40-23-15-38-26-24(41-31(34)35)16-39-25(23)26/h3-11,17,21-26,32H,12-16,28H2,1-2H3,(H,29,33)/t21-,22+,23-,24+,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497087
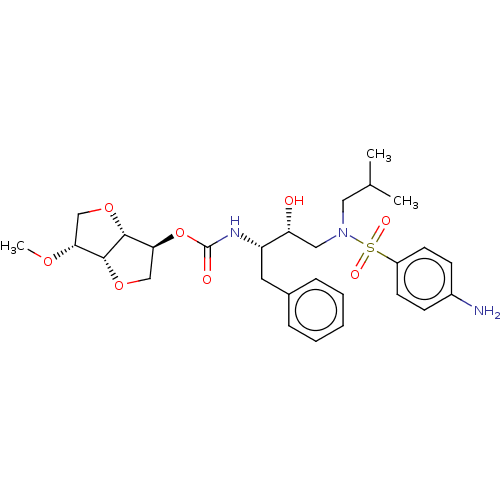
(CHEMBL3263094)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@@]1([H])OC[C@H]2OC |r| Show InChI InChI=1S/C28H39N3O8S/c1-18(2)14-31(40(34,35)21-11-9-20(29)10-12-21)15-23(32)22(13-19-7-5-4-6-8-19)30-28(33)39-25-17-38-26-24(36-3)16-37-27(25)26/h4-12,18,22-27,32H,13-17,29H2,1-3H3,(H,30,33)/t22-,23+,24+,25-,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497085
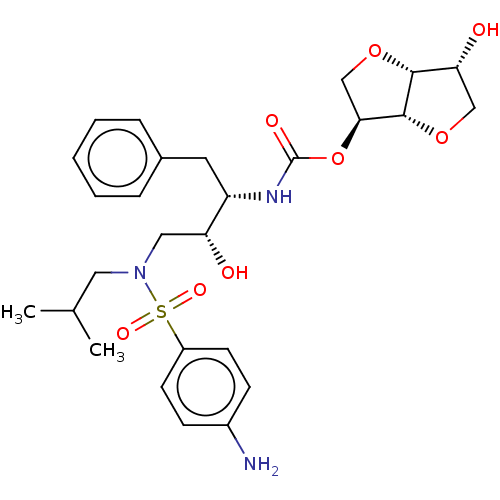
(CHEMBL3263093)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@@]1([H])OC[C@H]2O |r| Show InChI InChI=1S/C27H37N3O8S/c1-17(2)13-30(39(34,35)20-10-8-19(28)9-11-20)14-22(31)21(12-18-6-4-3-5-7-18)29-27(33)38-24-16-37-25-23(32)15-36-26(24)25/h3-11,17,21-26,31-32H,12-16,28H2,1-2H3,(H,29,33)/t21-,22+,23+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
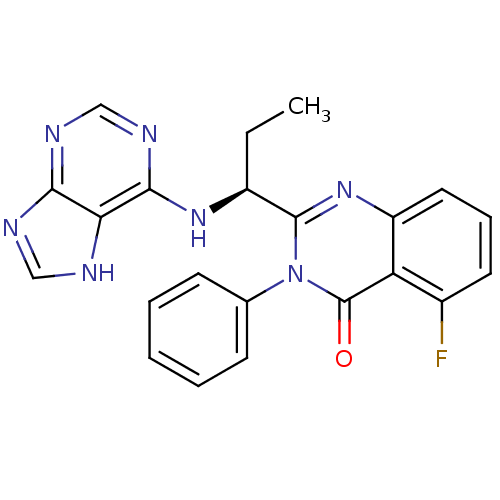
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50239835
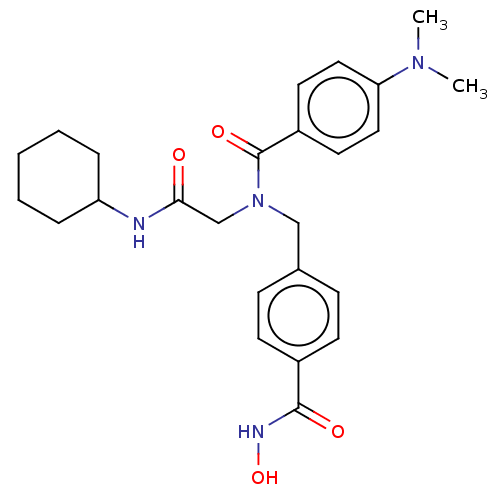
(CHEMBL4063658)Show SMILES CN(C)c1ccc(cc1)C(=O)N(CC(=O)NC1CCCCC1)Cc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C25H32N4O4/c1-28(2)22-14-12-20(13-15-22)25(32)29(17-23(30)26-21-6-4-3-5-7-21)16-18-8-10-19(11-9-18)24(31)27-33/h8-15,21,33H,3-7,16-17H2,1-2H3,(H,26,30)(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 fluorogenic peptide (379 to 382 residues) RHKKAc as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50531339

(CHEMBL4577299)Show SMILES ONC(=O)c1ccc(Cn2c3CCS(=O)(=O)Cc3c3ccccc23)cc1 Show InChI InChI=1S/C19H18N2O4S/c22-19(20-23)14-7-5-13(6-8-14)11-21-17-4-2-1-3-15(17)16-12-26(24,25)10-9-18(16)21/h1-8,23H,9-12H2,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic HDAC substrate incubated for 30 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579375
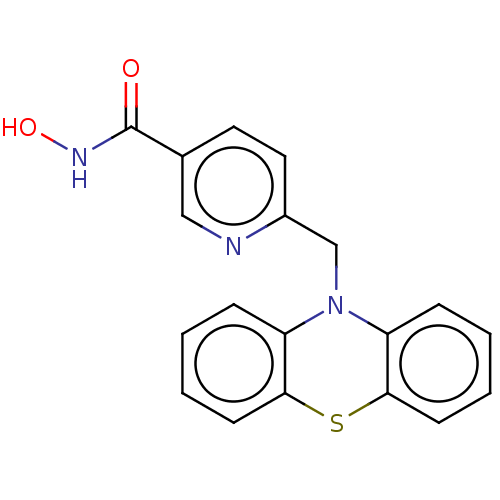
(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50531329
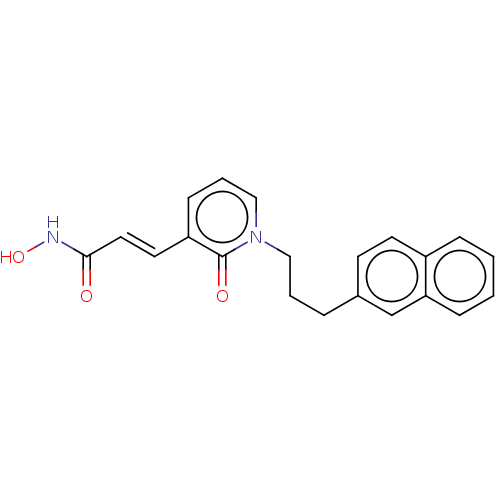
(CHEMBL4082995)Show InChI InChI=1S/C21H20N2O3/c24-20(22-26)12-11-18-8-4-14-23(21(18)25)13-3-5-16-9-10-17-6-1-2-7-19(17)15-16/h1-2,4,6-12,14-15,26H,3,5,13H2,(H,22,24)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full-length recombinant HDAC6 using fluorogenic peptide 79-382 (RHKK(Ac)) as substrate by fluorescence method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50252391
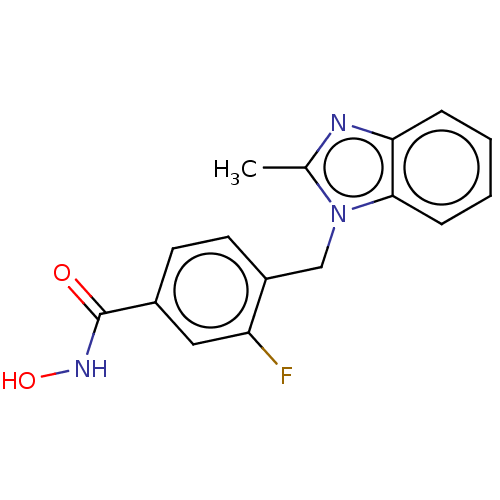
(CHEMBL4080014)Show InChI InChI=1S/C16H14FN3O2/c1-10-18-14-4-2-3-5-15(14)20(10)9-12-7-6-11(8-13(12)17)16(21)19-22/h2-8,22H,9H2,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 fluorogenic peptide (379 to 382 residues) RHKKAc as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50439674
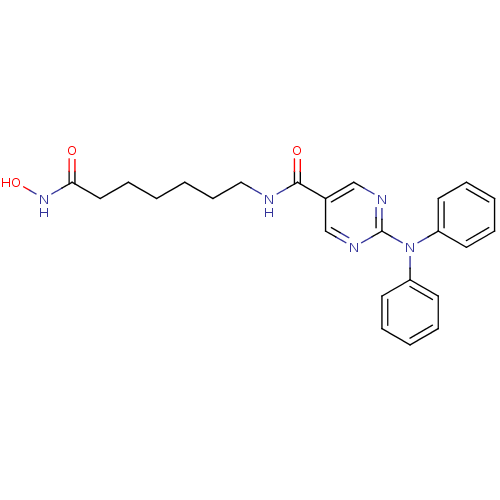
(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 (unknown origin) using FTS as substrate preincubated for 10 mins followed by substrate addition and shaken for 60 secs |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50382559
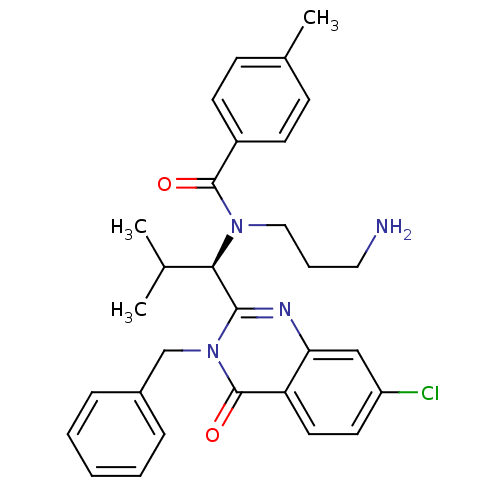
(ISPINESIB)Show SMILES CC(C)[C@@H](N(CCCN)C(=O)c1ccc(C)cc1)c1nc2cc(Cl)ccc2c(=O)n1Cc1ccccc1 Show InChI InChI=1S/C30H33ClN4O2/c1-20(2)27(34(17-7-16-32)29(36)23-12-10-21(3)11-13-23)28-33-26-18-24(31)14-15-25(26)30(37)35(28)19-22-8-5-4-6-9-22/h4-6,8-15,18,20,27H,7,16-17,19,32H2,1-3H3/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50548181
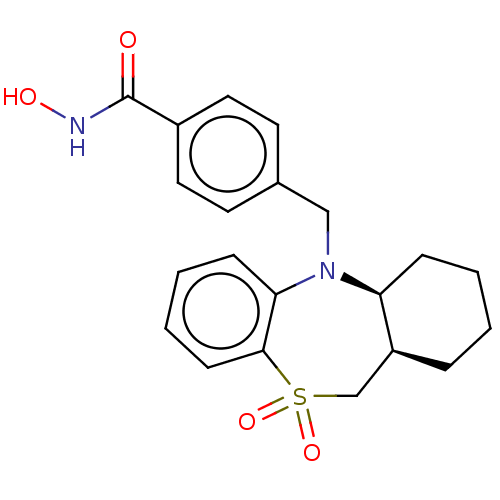
(CHEMBL4744479)Show SMILES [H][C@@]12CCCC[C@]1([H])N(Cc1ccc(cc1)C(=O)NO)c1ccccc1S(=O)(=O)C2 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50252392

(CHEMBL4069287)Show SMILES ONC(=O)c1ccc(Cn2c3CCS(=O)(=O)c3c3ccccc23)cc1 Show InChI InChI=1S/C18H16N2O4S/c21-18(19-22)13-7-5-12(6-8-13)11-20-15-4-2-1-3-14(15)17-16(20)9-10-25(17,23)24/h1-8,22H,9-11H2,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic HDAC substrate incubated for 30 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462367
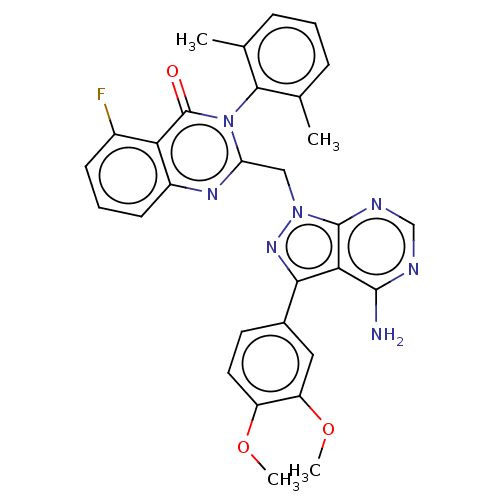
(CHEMBL4249477)Show SMILES COc1ccc(cc1OC)-c1nn(Cc2nc3cccc(F)c3c(=O)n2-c2c(C)cccc2C)c2ncnc(N)c12 |(83.53,-33.5,;81.99,-33.48,;81.23,-32.14,;79.69,-32.13,;78.94,-30.79,;79.73,-29.47,;81.26,-29.48,;82.03,-30.81,;83.57,-30.82,;84.33,-32.16,;78.97,-28.13,;79.87,-26.88,;78.96,-25.64,;78.95,-24.09,;77.61,-23.33,;76.29,-24.11,;74.95,-23.35,;73.63,-24.12,;72.3,-23.37,;72.28,-21.82,;73.61,-21.05,;73.6,-19.51,;74.94,-21.81,;76.27,-21.03,;76.26,-19.49,;77.6,-21.79,;78.93,-21.01,;80.27,-21.78,;80.27,-23.32,;81.6,-21.01,;81.59,-19.46,;80.24,-18.7,;78.92,-19.48,;77.58,-18.72,;77.5,-26.12,;76.16,-25.36,;74.83,-26.13,;74.83,-27.67,;76.16,-28.44,;76.16,-29.98,;77.5,-27.66,)| Show InChI InChI=1S/C30H26FN7O3/c1-16-7-5-8-17(2)27(16)38-23(35-20-10-6-9-19(31)24(20)30(38)39)14-37-29-25(28(32)33-15-34-29)26(36-37)18-11-12-21(40-3)22(13-18)41-4/h5-13,15H,14H2,1-4H3,(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462360
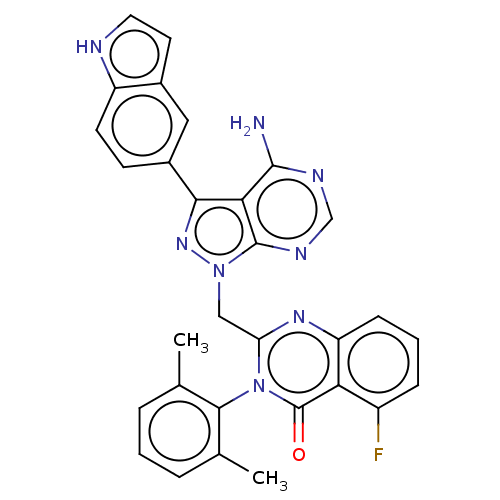
(CHEMBL4245221)Show SMILES Cc1cccc(C)c1-n1c(Cn2nc(-c3ccc4[nH]ccc4c3)c3c(N)ncnc23)nc2cccc(F)c2c1=O |(68.26,-24.08,;68.25,-22.54,;69.58,-21.77,;69.57,-20.23,;68.23,-19.46,;66.9,-20.24,;65.56,-19.49,;66.92,-21.78,;65.59,-22.55,;65.6,-24.1,;66.93,-24.86,;66.95,-26.4,;67.86,-27.64,;66.96,-28.89,;67.72,-30.24,;66.93,-31.55,;67.68,-32.89,;69.22,-32.91,;70.24,-34.07,;71.67,-33.46,;71.53,-31.92,;70.01,-31.57,;69.25,-30.24,;65.49,-28.43,;64.15,-29.21,;64.15,-30.75,;62.81,-28.43,;62.82,-26.89,;64.15,-26.12,;65.48,-26.88,;64.27,-24.88,;62.93,-24.11,;61.61,-24.88,;60.28,-24.13,;60.27,-22.59,;61.59,-21.81,;61.59,-20.27,;62.93,-22.57,;64.26,-21.79,;64.25,-20.25,)| Show InChI InChI=1S/C30H23FN8O/c1-16-5-3-6-17(2)27(16)39-23(36-22-8-4-7-20(31)24(22)30(39)40)14-38-29-25(28(32)34-15-35-29)26(37-38)19-9-10-21-18(13-19)11-12-33-21/h3-13,15,33H,14H2,1-2H3,(H2,32,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593
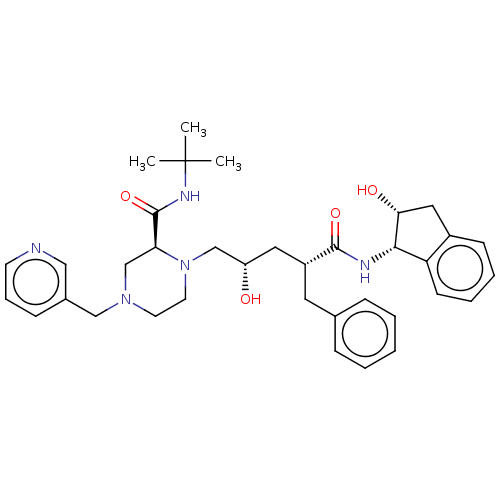
(CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate by fluorescence ass... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579375

(CHEMBL4858133) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50548182
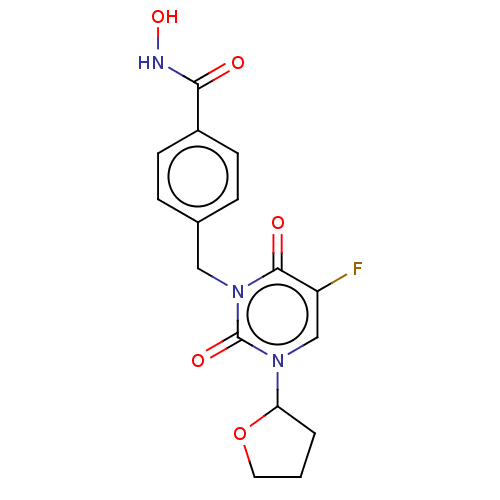
(CHEMBL4759780)Show SMILES ONC(=O)c1ccc(Cn2c(=O)c(F)cn(C3CCCO3)c2=O)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 fluorogenic peptide (379 to 382 residues) RHKKAc as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50538729

(CHEMBL4649617)Show InChI InChI=1S/C16H14N2O2S/c19-16(18-20)11-5-7-13(8-6-11)17-9-12-10-21-15-4-2-1-3-14(12)15/h1-8,10,17,20H,9H2,(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic HDAC substrate incubated for 30 mins by fluorimetry |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50380399

(CHEMBL2018302 | Tubastatin A | US10227295, Compoun...)Show InChI InChI=1S/C20H21N3O2/c1-22-11-10-19-17(13-22)16-4-2-3-5-18(16)23(19)12-14-6-8-15(9-7-14)20(24)21-25/h2-9,25H,10-13H2,1H3,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using p53 fluorogenic peptide (379 to 382 residues) RHKKAc as substrate by fluorescence-based assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462365

(CHEMBL4250311)Show SMILES Cc1ccccc1-n1c(Cn2nc(-c3ccc4[nH]ccc4c3)c3c(N)ncnc23)nc2cccc(F)c2c1=O |(54.76,-6.9,;54.76,-5.36,;56.09,-4.59,;56.08,-3.04,;54.74,-2.28,;53.41,-3.06,;53.43,-4.59,;52.1,-5.37,;52.1,-6.91,;53.44,-7.67,;53.45,-9.21,;54.37,-10.46,;53.47,-11.71,;54.22,-13.05,;53.43,-14.37,;54.19,-15.71,;55.73,-15.72,;56.75,-16.89,;58.17,-16.28,;58.03,-14.73,;56.52,-14.39,;55.75,-13.06,;52,-11.24,;50.66,-12.02,;50.66,-13.56,;49.32,-11.25,;49.32,-9.71,;50.65,-8.94,;51.99,-9.7,;50.78,-7.69,;49.44,-6.92,;48.12,-7.7,;46.79,-6.95,;46.77,-5.4,;48.1,-4.63,;48.09,-3.09,;49.43,-5.39,;50.76,-4.61,;50.76,-3.07,)| Show InChI InChI=1S/C29H21FN8O/c1-16-5-2-3-8-22(16)38-23(35-21-7-4-6-19(30)24(21)29(38)39)14-37-28-25(27(31)33-15-34-28)26(36-37)18-9-10-20-17(13-18)11-12-32-20/h2-13,15,32H,14H2,1H3,(H2,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50531329

(CHEMBL4082995)Show InChI InChI=1S/C21H20N2O3/c24-20(22-26)12-11-18-8-4-14-23(21(18)25)13-3-5-16-9-10-17-6-1-2-7-19(17)15-16/h1-2,4,6-12,14-15,26H,3,5,13H2,(H,22,24)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human full-length recombinant HDAC1 using p53 fluorogenic peptide (79 to 382 residues) (RHKK(Ac)) as substrate by fluorescence method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50161470

(6-(2-Mercapto-acetylamino)-hexanoic acid quinolin-...)Show InChI InChI=1S/C17H21N3O2S/c21-15(9-2-1-3-10-18-16(22)12-23)20-14-8-4-6-13-7-5-11-19-17(13)14/h4-8,11,23H,1-3,9-10,12H2,(H,18,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC6 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443437
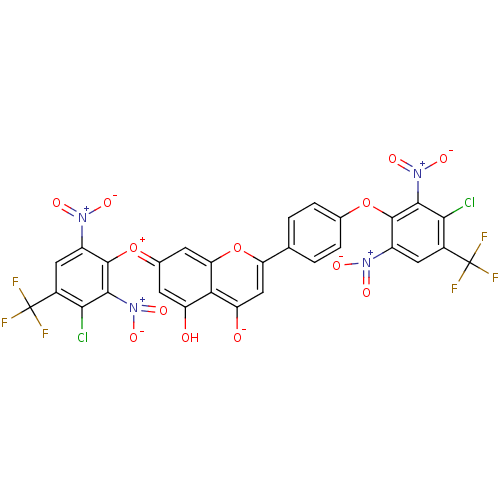
(CHEMBL3087419)Show SMILES Oc1c\c(cc2oc(cc([O-])c12)-c1ccc(Oc2c(cc(c(Cl)c2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc1)=[O+]/c1c(cc(c(Cl)c1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C29H10Cl2F6N4O13/c30-22-13(28(32,33)34)7-15(38(44)45)26(24(22)40(48)49)52-11-3-1-10(2-4-11)19-9-18(43)21-17(42)5-12(6-20(21)54-19)53-27-16(39(46)47)8-14(29(35,36)37)23(31)25(27)41(50)51/h1-9H,(H-,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462359
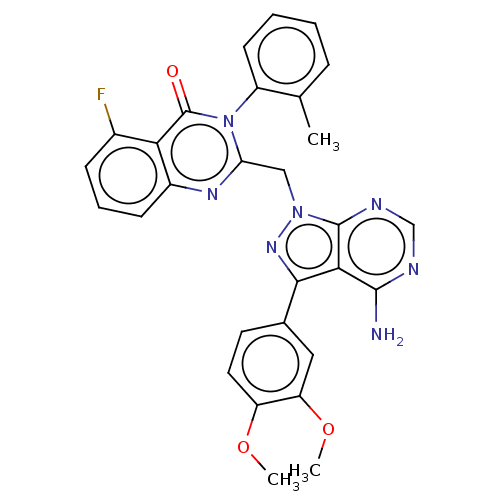
(CHEMBL4249850)Show SMILES COc1ccc(cc1OC)-c1nn(Cc2nc3cccc(F)c3c(=O)n2-c2ccccc2C)c2ncnc(N)c12 |(70.66,-16.38,;69.12,-16.36,;68.36,-15.02,;66.82,-15.01,;66.07,-13.67,;66.86,-12.35,;68.39,-12.35,;69.16,-13.69,;70.7,-13.7,;71.46,-15.04,;66.1,-11.01,;67,-9.75,;66.09,-8.51,;66.08,-6.97,;64.74,-6.21,;63.41,-6.99,;62.07,-6.22,;60.75,-6.99,;59.42,-6.24,;59.41,-4.7,;60.73,-3.92,;60.73,-2.38,;62.07,-4.68,;63.4,-3.9,;63.39,-2.36,;64.73,-4.66,;66.06,-3.89,;66.05,-2.35,;67.37,-1.57,;68.72,-2.34,;68.72,-3.88,;67.39,-4.66,;67.4,-6.2,;64.62,-9,;63.29,-8.23,;61.96,-9,;61.95,-10.55,;63.29,-11.32,;63.29,-12.86,;64.63,-10.54,)| Show InChI InChI=1S/C29H24FN7O3/c1-16-7-4-5-10-20(16)37-23(34-19-9-6-8-18(30)24(19)29(37)38)14-36-28-25(27(31)32-15-33-28)26(35-36)17-11-12-21(39-2)22(13-17)40-3/h4-13,15H,14H2,1-3H3,(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443438
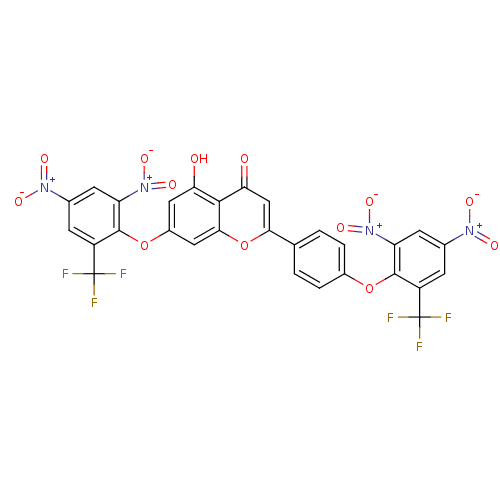
(CHEMBL3087418)Show SMILES Oc1cc(Oc2c(cc(cc2C(F)(F)F)[N+]([O-])=O)[N+]([O-])=O)cc2oc(cc(=O)c12)-c1ccc(Oc2c(cc(cc2C(F)(F)F)[N+]([O-])=O)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H12F6N4O13/c30-28(31,32)17-5-13(36(42)43)7-19(38(46)47)26(17)50-15-3-1-12(2-4-15)23-11-22(41)25-21(40)9-16(10-24(25)52-23)51-27-18(29(33,34)35)6-14(37(44)45)8-20(27)39(48)49/h1-11,40H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462363

(CHEMBL4241373)Show SMILES COc1ccc(cn1)-c1nn(Cc2nc3cccc(F)c3c(=O)n2-c2ccccc2C)c2ncnc(N)c12 |(15.11,-17.17,;13.57,-17.16,;12.81,-15.81,;13.6,-14.48,;12.84,-13.14,;11.31,-13.14,;10.52,-14.46,;11.27,-15.8,;10.55,-11.8,;11.45,-10.54,;10.54,-9.3,;10.53,-7.76,;9.19,-7,;7.86,-7.78,;6.52,-7.01,;5.2,-7.79,;3.87,-7.03,;3.86,-5.49,;5.18,-4.71,;5.18,-3.17,;6.52,-5.47,;7.85,-4.7,;7.84,-3.16,;9.18,-5.46,;10.51,-4.68,;10.49,-3.15,;11.82,-2.37,;13.16,-3.13,;13.17,-4.67,;11.84,-5.45,;11.85,-6.99,;9.07,-9.79,;7.74,-9.02,;6.41,-9.79,;6.4,-11.34,;7.74,-12.11,;7.74,-13.65,;9.08,-11.33,)| Show InChI InChI=1S/C27H21FN8O2/c1-15-6-3-4-9-19(15)36-20(33-18-8-5-7-17(28)22(18)27(36)37)13-35-26-23(25(29)31-14-32-26)24(34-35)16-10-11-21(38-2)30-12-16/h3-12,14H,13H2,1-2H3,(H2,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443436

(CHEMBL3087420)Show SMILES Oc1c(Oc2c(cc(cc2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)\c(cc2oc(cc([O-])c12)-c1ccccc1)=[O+]\c1c(cc(cc1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C29H12F6N4O13/c30-28(31,32)13-6-15(36(42)43)25(16(7-13)37(44)45)51-22-11-21-23(19(40)10-20(50-21)12-4-2-1-3-5-12)24(41)27(22)52-26-17(38(46)47)8-14(29(33,34)35)9-18(26)39(48)49/h1-11H,(H-,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510152
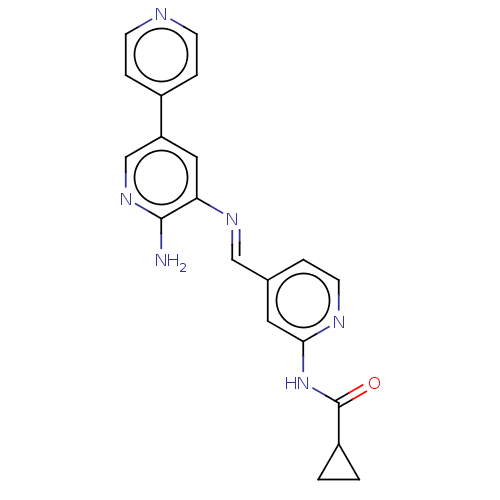
(CHEMBL4454448)Show SMILES Nc1ncc(cc1\N=C\c1ccnc(NC(=O)C2CC2)c1)-c1ccncc1 Show InChI InChI=1S/C20H18N6O/c21-19-17(10-16(12-25-19)14-4-6-22-7-5-14)24-11-13-3-8-23-18(9-13)26-20(27)15-1-2-15/h3-12,15H,1-2H2,(H2,21,25)(H,23,26,27)/b24-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50462366

(CHEMBL4243529)Show SMILES COc1ccc(cn1)-c1nn(Cc2nc3cccc(F)c3c(=O)n2-c2c(C)cccc2C)c2ncnc(N)c12 |(31.22,-36.01,;29.68,-35.99,;28.92,-34.65,;29.7,-33.32,;28.95,-31.98,;27.42,-31.98,;26.62,-33.3,;27.38,-34.64,;26.66,-30.64,;27.56,-29.38,;26.64,-28.14,;26.63,-26.6,;25.29,-25.84,;23.97,-26.62,;22.63,-25.85,;21.31,-26.62,;19.98,-25.87,;19.97,-24.33,;21.29,-23.55,;21.29,-22.01,;22.63,-24.31,;23.96,-23.53,;23.95,-21.99,;25.29,-24.29,;26.62,-23.52,;27.95,-24.29,;27.95,-25.83,;29.28,-23.51,;29.27,-21.97,;27.93,-21.21,;26.6,-21.99,;25.26,-21.23,;25.18,-28.63,;23.84,-27.86,;22.52,-28.63,;22.51,-30.18,;23.85,-30.95,;23.85,-32.49,;25.19,-30.17,)| Show InChI InChI=1S/C28H23FN8O2/c1-15-6-4-7-16(2)25(15)37-20(34-19-9-5-8-18(29)22(19)28(37)38)13-36-27-23(26(30)32-14-33-27)24(35-36)17-10-11-21(39-3)31-12-17/h4-12,14H,13H2,1-3H3,(H2,30,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using Biotin-S11S12 as substrate after 120 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC2 expressed in Sf9 insect cells using FTS as substrate preincubated for 10 mins followed by substrate addition and shaken for... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510144
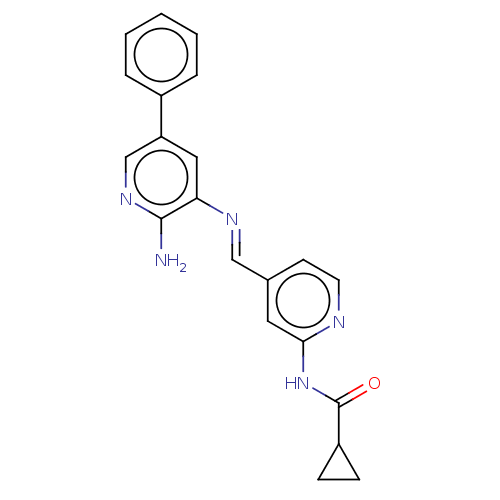
(CHEMBL4559169)Show SMILES Nc1ncc(cc1\N=C\c1ccnc(NC(=O)C2CC2)c1)-c1ccccc1 Show InChI InChI=1S/C21H19N5O/c22-20-18(11-17(13-25-20)15-4-2-1-3-5-15)24-12-14-8-9-23-19(10-14)26-21(27)16-6-7-16/h1-5,8-13,16H,6-7H2,(H2,22,25)(H,23,26,27)/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443425
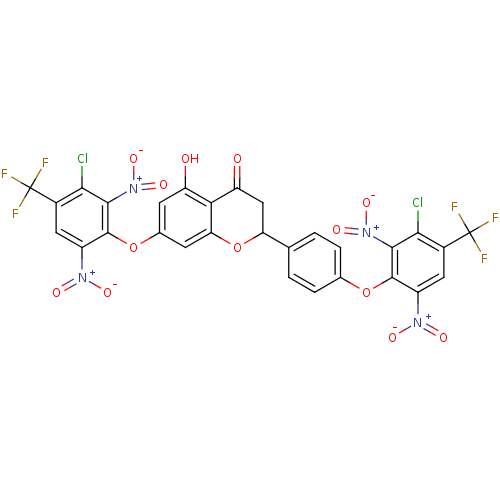
(CHEMBL3087430)Show SMILES Oc1cc(Oc2c(cc(c(Cl)c2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc2OC(CC(=O)c12)c1ccc(Oc2c(cc(c(Cl)c2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H12Cl2F6N4O13/c30-22-13(28(32,33)34)7-15(38(44)45)26(24(22)40(48)49)52-11-3-1-10(2-4-11)19-9-18(43)21-17(42)5-12(6-20(21)54-19)53-27-16(39(46)47)8-14(29(35,36)37)23(31)25(27)41(50)51/h1-8,19,42H,9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50579387
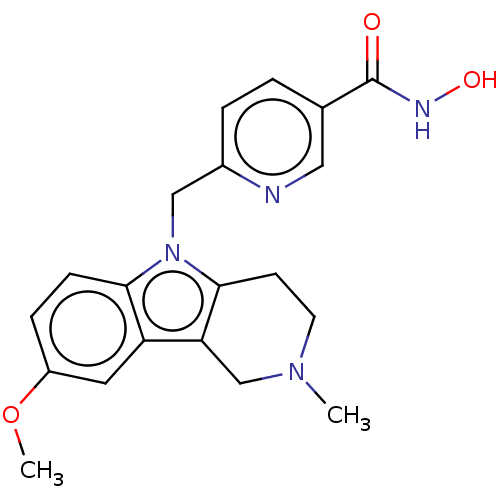
(CHEMBL4857648)Show SMILES COc1ccc2n(Cc3ccc(cn3)C(=O)NO)c3CCN(C)Cc3c2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 using fluorogenic substrate incubated for 1 hrs by fluorescence plate reader assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113821
BindingDB Entry DOI: 10.7270/Q2HX1HHW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM198121
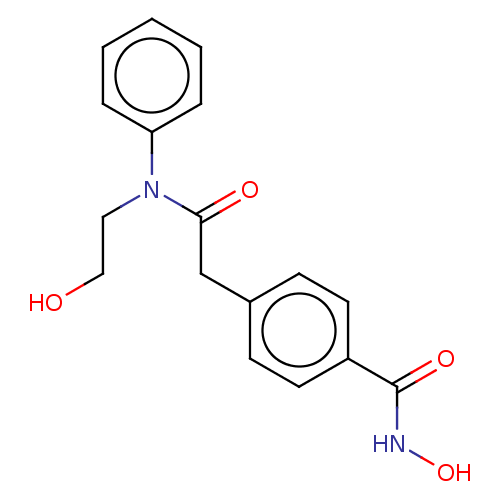
(HPOB)Show InChI InChI=1S/C17H18N2O4/c20-11-10-19(15-4-2-1-3-5-15)16(21)12-13-6-8-14(9-7-13)17(22)18-23/h1-9,20,23H,10-12H2,(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HDAC6 incubated for 30 mins by fluorescence method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50439674

(RICOLINOSTAT | US10858323, Compound 2 | US11207431...)Show SMILES ONC(=O)CCCCCCNC(=O)c1cnc(nc1)N(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H27N5O3/c30-22(28-32)15-9-1-2-10-16-25-23(31)19-17-26-24(27-18-19)29(20-11-5-3-6-12-20)21-13-7-4-8-14-21/h3-8,11-14,17-18,32H,1-2,9-10,15-16H2,(H,25,31)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC1 (unknown origin) using FTS as substrate preincubated for 10 mins followed by substrate addition and shaken for 60 secs |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443426
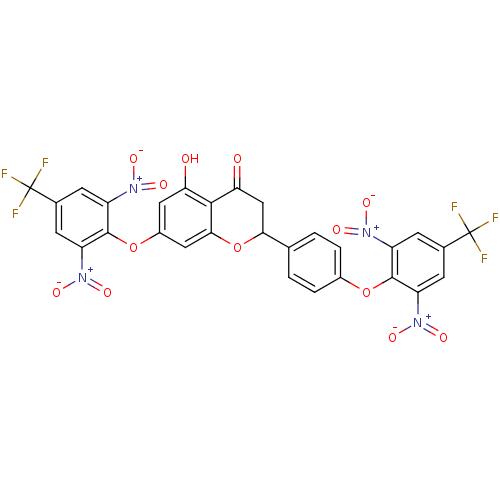
(CHEMBL3087429)Show SMILES Oc1cc(Oc2c(cc(cc2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc2OC(CC(=O)c12)c1ccc(Oc2c(cc(cc2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H14F6N4O13/c30-28(31,32)13-5-17(36(42)43)26(18(6-13)37(44)45)50-15-3-1-12(2-4-15)23-11-22(41)25-21(40)9-16(10-24(25)52-23)51-27-19(38(46)47)7-14(29(33,34)35)8-20(27)39(48)49/h1-10,23,40H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497085

(CHEMBL3263093)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@@]1([H])OC[C@H]2O |r| Show InChI InChI=1S/C27H37N3O8S/c1-17(2)13-30(39(34,35)20-10-8-19(28)9-11-20)14-22(31)21(12-18-6-4-3-5-7-18)29-27(33)38-24-16-37-25-23(32)15-36-26(24)25/h3-11,17,21-26,31-32H,12-16,28H2,1-2H3,(H,29,33)/t21-,22+,23+,24-,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate at 0.08 ng/ml by fl... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50462367

(CHEMBL4249477)Show SMILES COc1ccc(cc1OC)-c1nn(Cc2nc3cccc(F)c3c(=O)n2-c2c(C)cccc2C)c2ncnc(N)c12 |(83.53,-33.5,;81.99,-33.48,;81.23,-32.14,;79.69,-32.13,;78.94,-30.79,;79.73,-29.47,;81.26,-29.48,;82.03,-30.81,;83.57,-30.82,;84.33,-32.16,;78.97,-28.13,;79.87,-26.88,;78.96,-25.64,;78.95,-24.09,;77.61,-23.33,;76.29,-24.11,;74.95,-23.35,;73.63,-24.12,;72.3,-23.37,;72.28,-21.82,;73.61,-21.05,;73.6,-19.51,;74.94,-21.81,;76.27,-21.03,;76.26,-19.49,;77.6,-21.79,;78.93,-21.01,;80.27,-21.78,;80.27,-23.32,;81.6,-21.01,;81.59,-19.46,;80.24,-18.7,;78.92,-19.48,;77.58,-18.72,;77.5,-26.12,;76.16,-25.36,;74.83,-26.13,;74.83,-27.67,;76.16,-28.44,;76.16,-29.98,;77.5,-27.66,)| Show InChI InChI=1S/C30H26FN7O3/c1-16-7-5-8-17(2)27(16)38-23(35-20-10-6-9-19(31)24(20)30(38)39)14-37-29-25(28(32)33-15-34-29)26(36-37)18-11-12-21(40-3)22(13-18)41-4/h5-13,15H,14H2,1-4H3,(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using Biotin-S11S12 as substrate after 60 mins in presence of ATP by ADPGlo luminescence assay |
Eur J Med Chem 151: 9-17 (2018)
Article DOI: 10.1016/j.ejmech.2018.03.068
BindingDB Entry DOI: 10.7270/Q21N83S2 |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443427
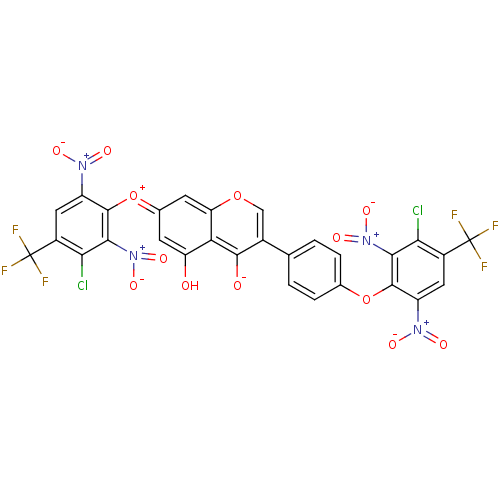
(CHEMBL3087428)Show SMILES Oc1c\c(cc2occ(c([O-])c12)-c1ccc(Oc2c(cc(c(Cl)c2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc1)=[O+]/c1c(cc(c(Cl)c1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C29H10Cl2F6N4O13/c30-21-14(28(32,33)34)7-16(38(44)45)26(23(21)40(48)49)53-11-3-1-10(2-4-11)13-9-52-19-6-12(5-18(42)20(19)25(13)43)54-27-17(39(46)47)8-15(29(35,36)37)22(31)24(27)41(50)51/h1-9H,(H-,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443429
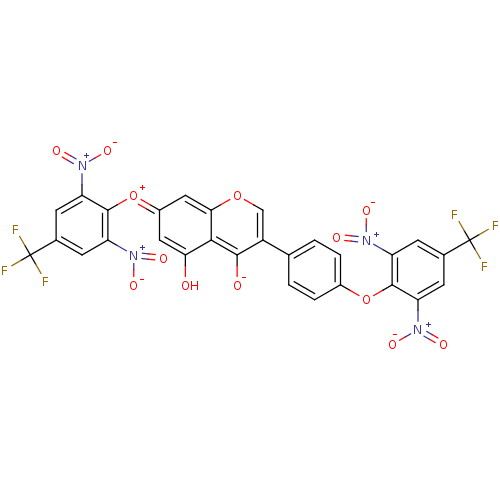
(CHEMBL3087426)Show SMILES Oc1c\c(cc2occ(-c3ccc(Oc4c(cc(cc4[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc3)c([O-])c12)=[O+]/c1c(cc(cc1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C29H12F6N4O13/c30-28(31,32)13-5-18(36(42)43)26(19(6-13)37(44)45)51-15-3-1-12(2-4-15)17-11-50-23-10-16(9-22(40)24(23)25(17)41)52-27-20(38(46)47)7-14(29(33,34)35)8-21(27)39(48)49/h1-11H,(H-,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50531329

(CHEMBL4082995)Show InChI InChI=1S/C21H20N2O3/c24-20(22-26)12-11-18-8-4-14-23(21(18)25)13-3-5-16-9-10-17-6-1-2-7-19(17)15-16/h1-2,4,6-12,14-15,26H,3,5,13H2,(H,22,24)/b12-11+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HDAC in human HeLa cell extracts using Fluor de lys as substrate incubated for 30 mins by fluorimetric analysis |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.10.040
BindingDB Entry DOI: 10.7270/Q25H7KV7 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497087

(CHEMBL3263094)Show SMILES [H][C@]12OC[C@H](OC(=O)N[C@@H](Cc3ccccc3)[C@H](O)CN(CC(C)C)S(=O)(=O)c3ccc(N)cc3)[C@@]1([H])OC[C@H]2OC |r| Show InChI InChI=1S/C28H39N3O8S/c1-18(2)14-31(40(34,35)21-11-9-20(29)10-12-21)15-23(32)22(13-19-7-5-4-6-8-19)30-28(33)39-25-17-38-26-24(36-3)16-37-27(25)26/h4-12,18,22-27,32H,13-17,29H2,1-3H3,(H,30,33)/t22-,23+,24+,25-,26+,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate at 0.08 ng/ml by fl... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50510145
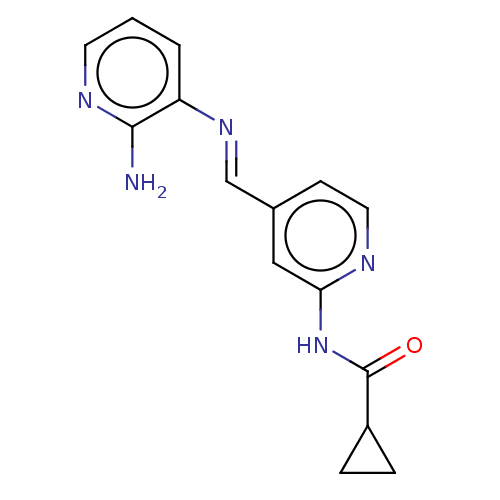
(CHEMBL4467668)Show InChI InChI=1S/C15H15N5O/c16-14-12(2-1-6-18-14)19-9-10-5-7-17-13(8-10)20-15(21)11-3-4-11/h1-2,5-9,11H,3-4H2,(H2,16,18)(H,17,20,21)/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of GSK-3beta (unknown origin) using FAM-labeled peptide as substrate preincubated for 10 mins followed by substrate addition by caliper as... |
Eur J Med Chem 167: 211-225 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.001
BindingDB Entry DOI: 10.7270/Q26976WC |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50443430
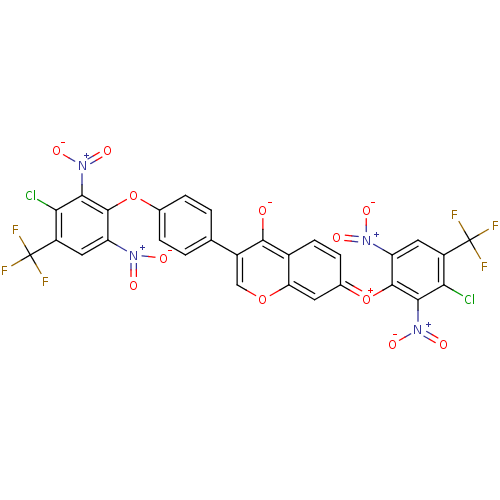
(CHEMBL3087425)Show SMILES [O-]c1c(coc2c\c(ccc12)=[O+]\c1c(cc(c(Cl)c1[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)-c1ccc(Oc2c(cc(c(Cl)c2[N+]([O-])=O)C(F)(F)F)[N+]([O-])=O)cc1 Show InChI InChI=1S/C29H10Cl2F6N4O12/c30-21-16(28(32,33)34)8-18(38(43)44)26(23(21)40(47)48)52-12-3-1-11(2-4-12)15-10-51-20-7-13(5-6-14(20)25(15)42)53-27-19(39(45)46)9-17(29(35,36)37)22(31)24(27)41(49)50/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged microtubule-stimulated KSP motor domain (1 to 369) ATPase activity (unknown origin) preincubated for 30 mins foll... |
Eur J Med Chem 70: 427-33 (2013)
Article DOI: 10.1016/j.ejmech.2013.09.042
BindingDB Entry DOI: 10.7270/Q289179H |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50497090

(CHEMBL3263092)Show SMILES [H][C@]12OC[C@@H](O[N+]([O-])=O)[C@@]1([H])OC[C@@H]2OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C27H36N4O10S/c1-17(2)13-30(42(36,37)20-10-8-19(28)9-11-20)14-22(32)21(12-18-6-4-3-5-7-18)29-27(33)40-23-15-38-26-24(41-31(34)35)16-39-25(23)26/h3-11,17,21-26,32H,12-16,28H2,1-2H3,(H,29,33)/t21-,22+,23-,24+,25+,26+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 77.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University
Curated by ChEMBL
| Assay Description
Inhibition of purified HIV-1 protease using [Arg-Glu(EDANS)-Ser-Gin-Asn-Tyr-Ile-Val-Gin-Lys(dabcyl)-Arg) as fluorogenic substrate at 0.08 ng/ml by fl... |
Bioorg Med Chem Lett 24: 2465-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.008
BindingDB Entry DOI: 10.7270/Q20C4ZRC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data