 Found 206 hits with Last Name = 'lockman' and Initial = 'jw'
Found 206 hits with Last Name = 'lockman' and Initial = 'jw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347432

(CHEMBL1801863)Show SMILES Cn1c(CN2CCN(CCCN)CC2)nc2cc(Cl)c(CN(CC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C34H39ClN8O2/c1-3-13-43(28-9-7-26(8-10-28)33(44)38-22-25-6-4-12-37-21-25)23-27-19-29-31(20-30(27)35)39-32(40(2)34(29)45)24-42-17-15-41(16-18-42)14-5-11-36/h1,4,6-10,12,19-21H,5,11,13-18,22-24,36H2,2H3,(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50117198
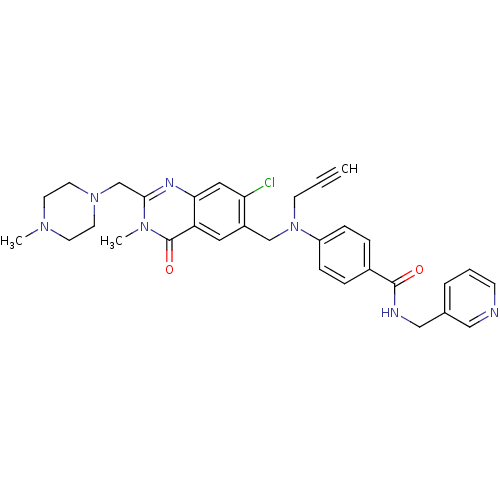
(4-{[7-Chloro-3-methyl-2-(4-methyl-piperazin-1-ylme...)Show SMILES CN1CCN(Cc2nc3cc(Cl)c(CN(CC#C)c4ccc(cc4)C(=O)NCc4cccnc4)cc3c(=O)n2C)CC1 Show InChI InChI=1S/C32H34ClN7O2/c1-4-12-40(26-9-7-24(8-10-26)31(41)35-20-23-6-5-11-34-19-23)21-25-17-27-29(18-28(25)33)36-30(38(3)32(27)42)22-39-15-13-37(2)14-16-39/h1,5-11,17-19H,12-16,20-22H2,2-3H3,(H,35,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347433

(CHEMBL1801864)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1cc2c(cc1Cl)nnn(-[#6])c2=O)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C27H27ClN6O2/c1-18(2)10-12-34(17-21-13-23-25(14-24(21)28)31-32-33(3)27(23)36)22-8-6-20(7-9-22)26(35)30-16-19-5-4-11-29-15-19/h4-11,13-15H,12,16-17H2,1-3H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50067584

((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N3O3S2/c1-29-10-9-19(21(26)27)24-20(25)17-8-7-16(23-12-15(22)13-28)11-18(17)14-5-3-2-4-6-14/h2-8,11,15,19,23,28H,9-10,12-13,22H2,1H3,(H,24,25)(H,26,27)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian Farnesyltransferase |
Bioorg Med Chem Lett 11: 761-4 (2001)
BindingDB Entry DOI: 10.7270/Q26W99CH |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50067584

((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N3O3S2/c1-29-10-9-19(21(26)27)24-20(25)17-8-7-16(23-12-15(22)13-28)11-18(17)14-5-3-2-4-6-14/h2-8,11,15,19,23,28H,9-10,12-13,22H2,1H3,(H,24,25)(H,26,27)/t15-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347427
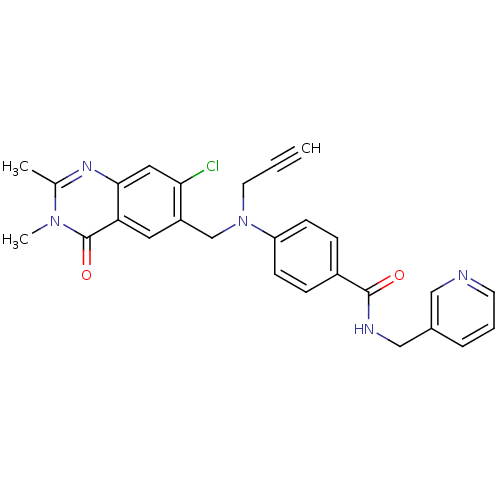
(CHEMBL1801934)Show SMILES Cc1nc2cc(Cl)c(CN(CC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c(=O)n1C Show InChI InChI=1S/C27H24ClN5O2/c1-4-12-33(17-21-13-23-25(14-24(21)28)31-18(2)32(3)27(23)35)22-9-7-20(8-10-22)26(34)30-16-19-6-5-11-29-15-19/h1,5-11,13-15H,12,16-17H2,2-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50067584

((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N3O3S2/c1-29-10-9-19(21(26)27)24-20(25)17-8-7-16(23-12-15(22)13-28)11-18(17)14-5-3-2-4-6-14/h2-8,11,15,19,23,28H,9-10,12-13,22H2,1H3,(H,24,25)(H,26,27)/t15-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells |
J Med Chem 45: 177-88 (2001)
BindingDB Entry DOI: 10.7270/Q22F7MQ7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347431

(CHEMBL1801862)Show SMILES Cn1c(CO)nc2cc(Cl)c(CN(CC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C27H24ClN5O3/c1-3-11-33(16-20-12-22-24(13-23(20)28)31-25(17-34)32(2)27(22)36)21-8-6-19(7-9-21)26(35)30-15-18-5-4-10-29-14-18/h1,4-10,12-14,34H,11,15-17H2,2H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16182
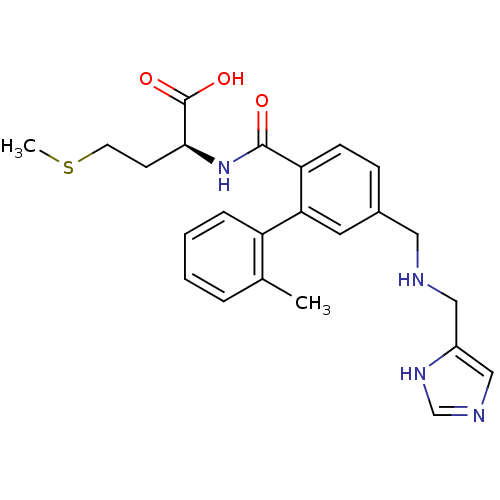
((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CNCc2cnc[nH]2)cc1-c1ccccc1C)C(O)=O |r| Show InChI InChI=1S/C24H28N4O3S/c1-16-5-3-4-6-19(16)21-11-17(12-25-13-18-14-26-15-27-18)7-8-20(21)23(29)28-22(24(30)31)9-10-32-2/h3-8,11,14-15,22,25H,9-10,12-13H2,1-2H3,(H,26,27)(H,28,29)(H,30,31)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM16182

((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CNCc2cnc[nH]2)cc1-c1ccccc1C)C(O)=O |r| Show InChI InChI=1S/C24H28N4O3S/c1-16-5-3-4-6-19(16)21-11-17(12-25-13-18-14-26-15-27-18)7-8-20(21)23(29)28-22(24(30)31)9-10-32-2/h3-8,11,14-15,22,25H,9-10,12-13H2,1-2H3,(H,26,27)(H,28,29)(H,30,31)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian Farnesyltransferase |
Bioorg Med Chem Lett 11: 761-4 (2001)
BindingDB Entry DOI: 10.7270/Q26W99CH |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347394
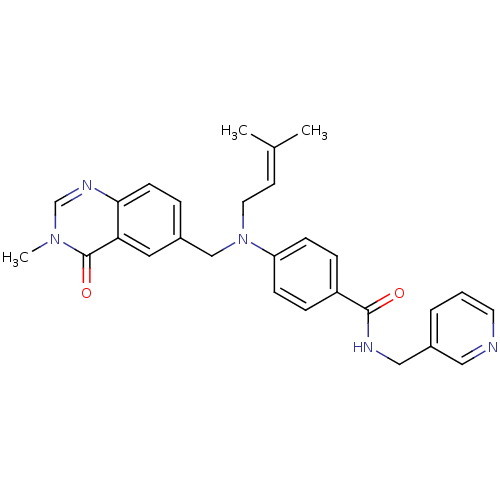
(CHEMBL1801566)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1ccc2ncn(-[#6])c(=O)c2c1)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C28H29N5O2/c1-20(2)12-14-33(18-21-6-11-26-25(15-21)28(35)32(3)19-31-26)24-9-7-23(8-10-24)27(34)30-17-22-5-4-13-29-16-22/h4-13,15-16,19H,14,17-18H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347407

(CHEMBL1801561)Show SMILES Clc1ccccc1CN(CC1CC1)c1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C24H24ClN3O/c25-23-6-2-1-5-21(23)17-28(16-18-7-8-18)22-11-9-20(10-12-22)24(29)27-15-19-4-3-13-26-14-19/h1-6,9-14,18H,7-8,15-17H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347415

(CHEMBL1801552)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1ccc2nc(-[#6])n(-[#6])c(=O)c2c1)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C29H31N5O2/c1-20(2)13-15-34(19-22-7-12-27-26(16-22)29(36)33(4)21(3)32-27)25-10-8-24(9-11-25)28(35)31-18-23-6-5-14-30-17-23/h5-14,16-17H,15,18-19H2,1-4H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347391
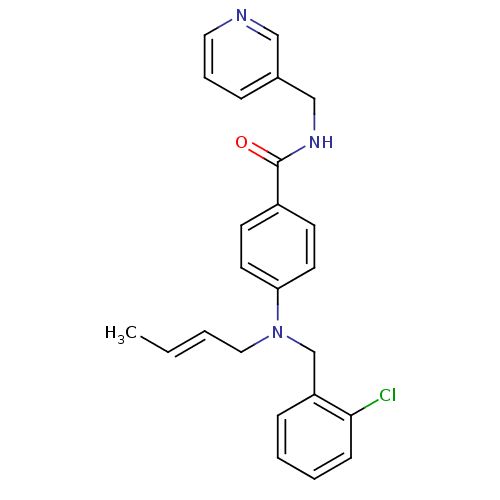
(CHEMBL1801563)Show SMILES C\C=C\CN(Cc1ccccc1Cl)c1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C24H24ClN3O/c1-2-3-15-28(18-21-8-4-5-9-23(21)25)22-12-10-20(11-13-22)24(29)27-17-19-7-6-14-26-16-19/h2-14,16H,15,17-18H2,1H3,(H,27,29)/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50067584

((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C21H27N3O3S2/c1-29-10-9-19(21(26)27)24-20(25)17-8-7-16(23-12-15(22)13-28)11-18(17)14-5-3-2-4-6-14/h2-8,11,15,19,23,28H,9-10,12-13,22H2,1H3,(H,24,25)(H,26,27)/t15-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM16182

((2S)-2-[(4-{[(1H-imidazol-4-ylmethyl)amino]methyl}...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CNCc2cnc[nH]2)cc1-c1ccccc1C)C(O)=O |r| Show InChI InChI=1S/C24H28N4O3S/c1-16-5-3-4-6-19(16)21-11-17(12-25-13-18-14-26-15-27-18)7-8-20(21)23(29)28-22(24(30)31)9-10-32-2/h3-8,11,14-15,22,25H,9-10,12-13H2,1-2H3,(H,26,27)(H,28,29)(H,30,31)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347406
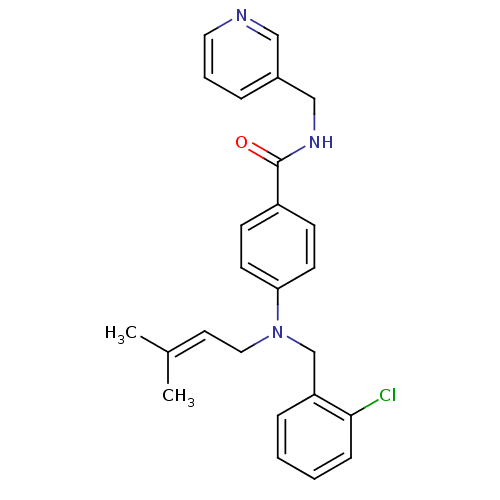
(CHEMBL1801562)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1ccccc1Cl)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C25H26ClN3O/c1-19(2)13-15-29(18-22-7-3-4-8-24(22)26)23-11-9-21(10-12-23)25(30)28-17-20-6-5-14-27-16-20/h3-14,16H,15,17-18H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347395
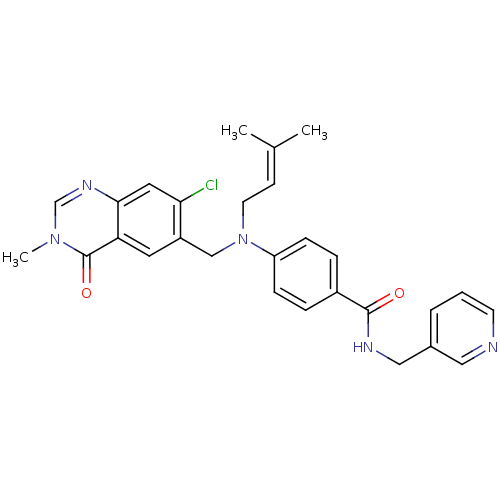
(CHEMBL1801567)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1cc2c(cc1Cl)ncn(-[#6])c2=O)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C28H28ClN5O2/c1-19(2)10-12-34(17-22-13-24-26(14-25(22)29)32-18-33(3)28(24)36)23-8-6-21(7-9-23)27(35)31-16-20-5-4-11-30-15-20/h4-11,13-15,18H,12,16-17H2,1-3H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347401
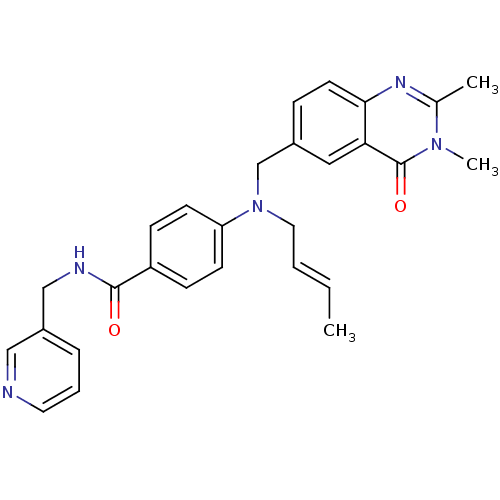
(CHEMBL1801855)Show SMILES C\C=C\CN(Cc1ccc2nc(C)n(C)c(=O)c2c1)c1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C28H29N5O2/c1-4-5-15-33(19-21-8-13-26-25(16-21)28(35)32(3)20(2)31-26)24-11-9-23(10-12-24)27(34)30-18-22-7-6-14-29-17-22/h4-14,16-17H,15,18-19H2,1-3H3,(H,30,34)/b5-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347441
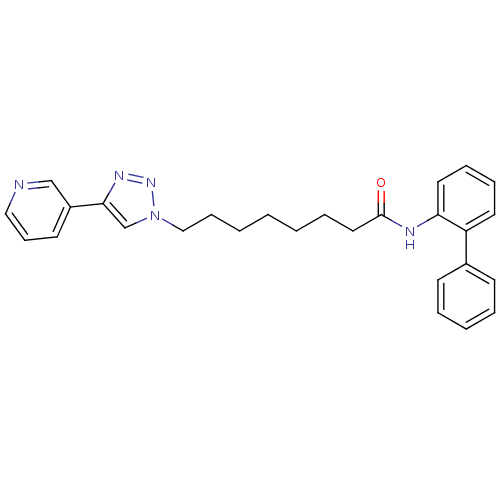
(CHEMBL568305)Show SMILES O=C(CCCCCCCn1cc(nn1)-c1cccnc1)Nc1ccccc1-c1ccccc1 Show InChI InChI=1S/C27H29N5O/c33-27(29-25-16-9-8-15-24(25)22-12-5-4-6-13-22)17-7-2-1-3-10-19-32-21-26(30-31-32)23-14-11-18-28-20-23/h4-6,8-9,11-16,18,20-21H,1-3,7,10,17,19H2,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-mediated decrease in cellular NAD level |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347424
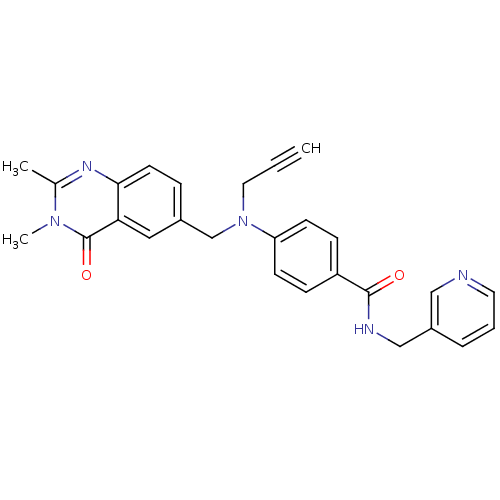
(CHEMBL1801937)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c(=O)n1C Show InChI InChI=1S/C27H25N5O2/c1-4-14-32(18-20-7-12-25-24(15-20)27(34)31(3)19(2)30-25)23-10-8-22(9-11-23)26(33)29-17-21-6-5-13-28-16-21/h1,5-13,15-16H,14,17-18H2,2-3H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347430
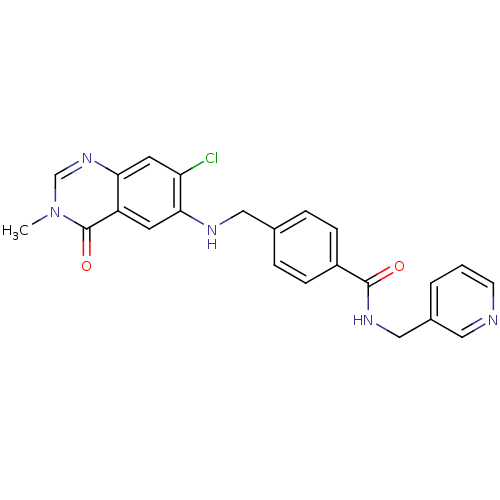
(CHEMBL1801861)Show SMILES Cn1cnc2cc(Cl)c(NCc3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C23H20ClN5O2/c1-29-14-28-20-10-19(24)21(9-18(20)23(29)31)26-12-15-4-6-17(7-5-15)22(30)27-13-16-3-2-8-25-11-16/h2-11,14,26H,12-13H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347408
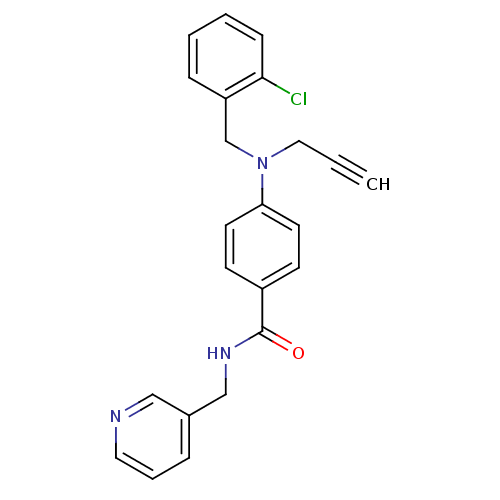
(CHEMBL1801560)Show SMILES Clc1ccccc1CN(CC#C)c1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C23H20ClN3O/c1-2-14-27(17-20-7-3-4-8-22(20)24)21-11-9-19(10-12-21)23(28)26-16-18-6-5-13-25-15-18/h1,3-13,15H,14,16-17H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50138762

((S)-2-[(5-{[Bis-(1H-imidazol-4-ylmethyl)-amino]-me...)Show SMILES CSCC[C@H](NC(=O)c1ccc(CN(Cc2cnc[nH]2)Cc2cnc[nH]2)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C28H32N6O3S/c1-19-5-3-4-6-23(19)25-11-20(7-8-24(25)27(35)33-26(28(36)37)9-10-38-2)14-34(15-21-12-29-17-31-21)16-22-13-30-18-32-22/h3-8,11-13,17-18,26H,9-10,14-16H2,1-2H3,(H,29,31)(H,30,32)(H,33,35)(H,36,37)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347442
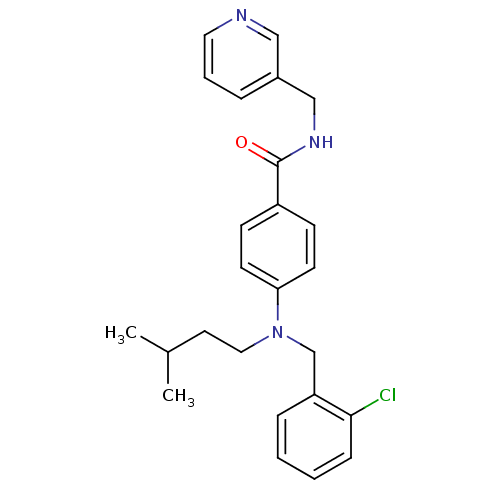
(CHEMBL1801559)Show SMILES CC(C)CCN(Cc1ccccc1Cl)c1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C25H28ClN3O/c1-19(2)13-15-29(18-22-7-3-4-8-24(22)26)23-11-9-21(10-12-23)25(30)28-17-20-6-5-14-27-16-20/h3-12,14,16,19H,13,15,17-18H2,1-2H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50138763
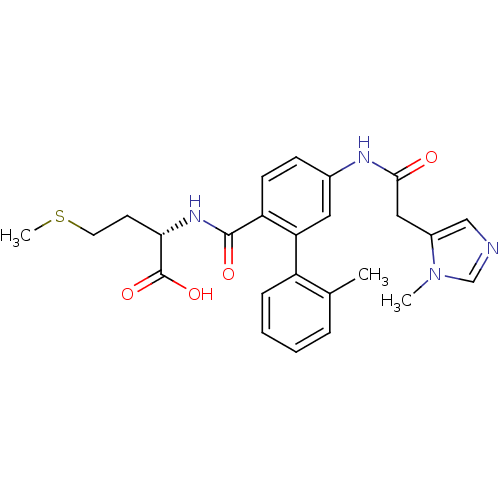
((S)-2-({2'-Methyl-5-[2-(3-methyl-3H-imidazol-4-yl)...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NC(=O)Cc2cncn2C)cc1-c1ccccc1C)C(O)=O Show InChI InChI=1S/C25H28N4O4S/c1-16-6-4-5-7-19(16)21-12-17(27-23(30)13-18-14-26-15-29(18)2)8-9-20(21)24(31)28-22(25(32)33)10-11-34-3/h4-9,12,14-15,22H,10-11,13H2,1-3H3,(H,27,30)(H,28,31)(H,32,33)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50097809
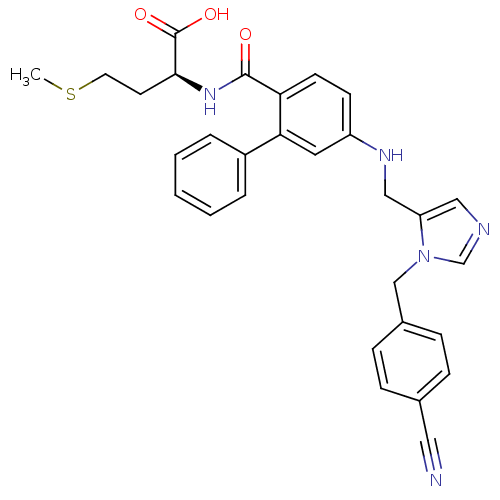
((S)-2-[(5-{[3-(4-Cyano-benzyl)-3H-imidazol-4-ylmet...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NCc2cncn2Cc2ccc(cc2)C#N)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C30H29N5O3S/c1-39-14-13-28(30(37)38)34-29(36)26-12-11-24(15-27(26)23-5-3-2-4-6-23)33-18-25-17-32-20-35(25)19-22-9-7-21(16-31)8-10-22/h2-12,15,17,20,28,33H,13-14,18-19H2,1H3,(H,34,36)(H,37,38)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian Farnesyltransferase |
Bioorg Med Chem Lett 11: 761-4 (2001)
BindingDB Entry DOI: 10.7270/Q26W99CH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50042034
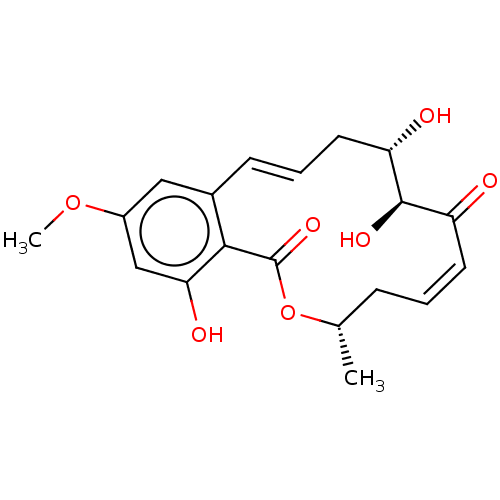
((5Z)-7-Oxozeaenol | 5Z-7-Oxozeaenol | CHEBI:67559 ...)Show SMILES COc1cc(O)c2c(c1)\C=C\C[C@H](O)[C@H](O)C(=O)\C=C/C[C@H](C)OC2=O |r,c:19,t:10| Show InChI InChI=1S/C19H22O7/c1-11-5-3-7-14(20)18(23)15(21)8-4-6-12-9-13(25-2)10-16(22)17(12)19(24)26-11/h3-4,6-7,9-11,15,18,21-23H,5,8H2,1-2H3/b6-4+,7-3-/t11-,15-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant TAK1-TAB1 assessed as [33P]gamma-ATP incorporation into substrate histone H1 peptide by filter plate assay |
Bioorg Med Chem Lett 21: 1724-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.077
BindingDB Entry DOI: 10.7270/Q2988794 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50097810

((S)-2-({5-[(3-Biphenyl-4-ylmethyl-3H-imidazol-4-yl...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NCc2cncn2Cc2ccc(cc2)-c2ccccc2)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C35H34N4O3S/c1-43-19-18-33(35(41)42)38-34(40)31-17-16-29(20-32(31)28-10-6-3-7-11-28)37-22-30-21-36-24-39(30)23-25-12-14-27(15-13-25)26-8-4-2-5-9-26/h2-17,20-21,24,33,37H,18-19,22-23H2,1H3,(H,38,40)(H,41,42)/t33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase at 50 nM |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50191935
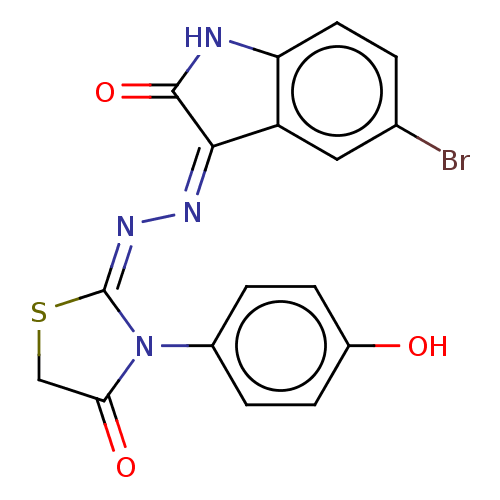
(CHEMBL1761718)Show SMILES Oc1ccc(cc1)N1C(=O)CS\C1=N/N=C1\C(=O)Nc2ccc(Br)cc12 Show InChI InChI=1S/C17H11BrN4O3S/c18-9-1-6-13-12(7-9)15(16(25)19-13)20-21-17-22(14(24)8-26-17)10-2-4-11(23)5-3-10/h1-7,23H,8H2,(H,19,20,25)/b21-17- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant TAK1-TAB1 assessed as [33P]gamma-ATP incorporation into substrate histone H1 peptide by filter plate assay |
Bioorg Med Chem Lett 21: 1724-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.077
BindingDB Entry DOI: 10.7270/Q2988794 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347426
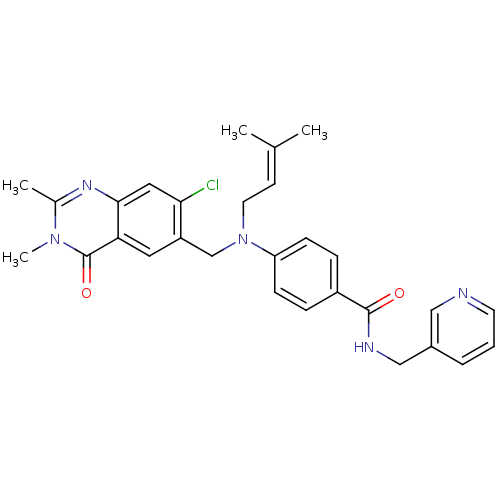
(CHEMBL1801935)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#7](-[#6]-c1cc2c(cc1Cl)nc(-[#6])n(-[#6])c2=O)-c1ccc(cc1)-[#6](=O)-[#7]-[#6]-c1cccnc1 Show InChI InChI=1S/C29H30ClN5O2/c1-19(2)11-13-35(18-23-14-25-27(15-26(23)30)33-20(3)34(4)29(25)37)24-9-7-22(8-10-24)28(36)32-17-21-6-5-12-31-16-21/h5-12,14-16H,13,17-18H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347429
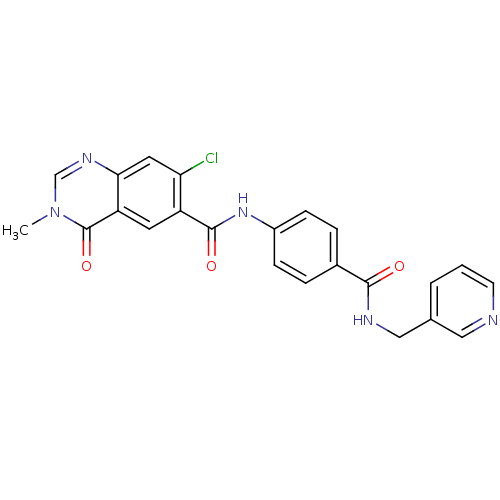
(CHEMBL1801860)Show SMILES Cn1cnc2cc(Cl)c(cc2c1=O)C(=O)Nc1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C23H18ClN5O3/c1-29-13-27-20-10-19(24)17(9-18(20)23(29)32)22(31)28-16-6-4-15(5-7-16)21(30)26-12-14-3-2-8-25-11-14/h2-11,13H,12H2,1H3,(H,26,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347393
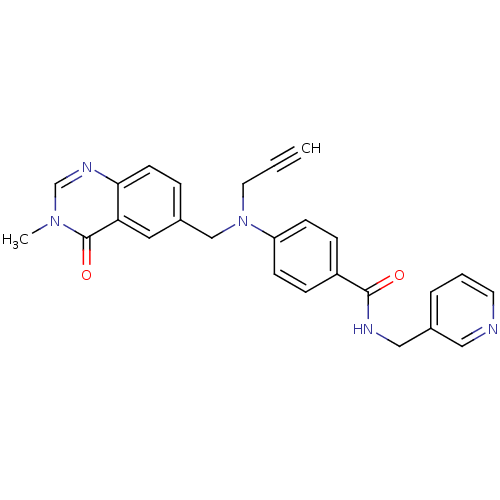
(CHEMBL1801565)Show SMILES Cn1cnc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C26H23N5O2/c1-3-13-31(17-19-6-11-24-23(14-19)26(33)30(2)18-29-24)22-9-7-21(8-10-22)25(32)28-16-20-5-4-12-27-15-20/h1,4-12,14-15,18H,13,16-17H2,2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347398

(CHEMBL1801570)Show SMILES Cn1cnc2cc(Cl)c(CN(CCC#C)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C27H24ClN5O2/c1-3-4-12-33(17-21-13-23-25(14-24(21)28)31-18-32(2)27(23)35)22-9-7-20(8-10-22)26(34)30-16-19-6-5-11-29-15-19/h1,5-11,13-15,18H,4,12,16-17H2,2H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347396
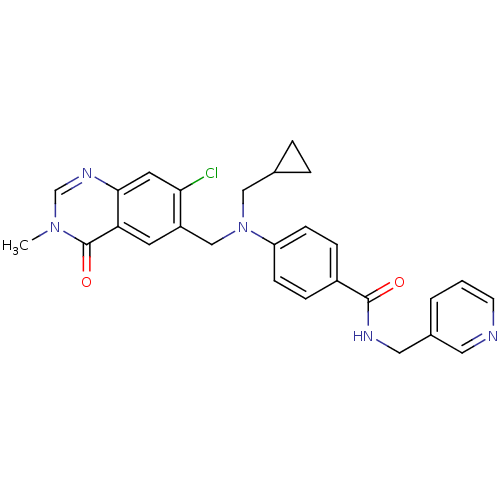
(CHEMBL1801568)Show SMILES Cn1cnc2cc(Cl)c(CN(CC3CC3)c3ccc(cc3)C(=O)NCc3cccnc3)cc2c1=O Show InChI InChI=1S/C27H26ClN5O2/c1-32-17-31-25-12-24(28)21(11-23(25)27(32)35)16-33(15-18-4-5-18)22-8-6-20(7-9-22)26(34)30-14-19-3-2-10-29-13-19/h2-3,6-13,17-18H,4-5,14-16H2,1H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347411

(CHEMBL1801556)Show InChI InChI=1S/C23H24ClN3O/c1-2-14-27(17-20-7-3-4-8-22(20)24)21-11-9-19(10-12-21)23(28)26-16-18-6-5-13-25-15-18/h3-13,15H,2,14,16-17H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50097814
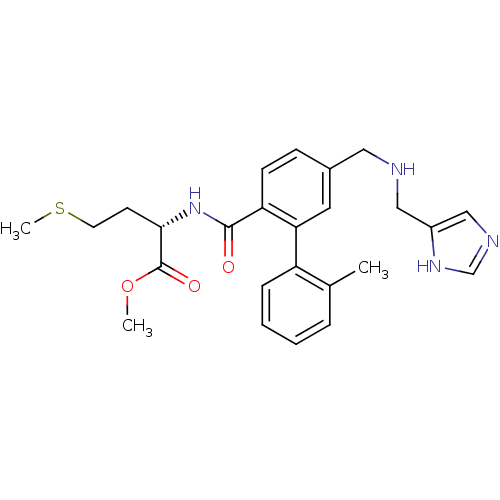
((S)-2-[(5-{[(1H-Imidazol-4-ylmethyl)-amino]-methyl...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)c1ccc(CNCc2cnc[nH]2)cc1-c1ccccc1C Show InChI InChI=1S/C25H30N4O3S/c1-17-6-4-5-7-20(17)22-12-18(13-26-14-19-15-27-16-28-19)8-9-21(22)24(30)29-23(10-11-33-3)25(31)32-2/h4-9,12,15-16,23,26H,10-11,13-14H2,1-3H3,(H,27,28)(H,29,30)/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian H-Ras processing in NIH 3T3 cells |
Bioorg Med Chem Lett 11: 761-4 (2001)
BindingDB Entry DOI: 10.7270/Q26W99CH |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50108065
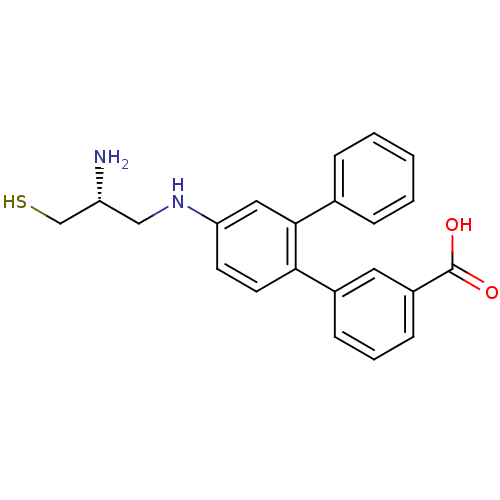
(4'-(2-Amino-3-mercapto-propylamino)-[1,1';2',1'']t...)Show SMILES N[C@@H](CS)CNc1ccc(-c2cccc(c2)C(O)=O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H22N2O2S/c23-18(14-27)13-24-19-9-10-20(16-7-4-8-17(11-16)22(25)26)21(12-19)15-5-2-1-3-6-15/h1-12,18,24,27H,13-14,23H2,(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells |
J Med Chem 45: 177-88 (2001)
BindingDB Entry DOI: 10.7270/Q22F7MQ7 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1
(Homo sapiens (Human)) | BDBM50191939
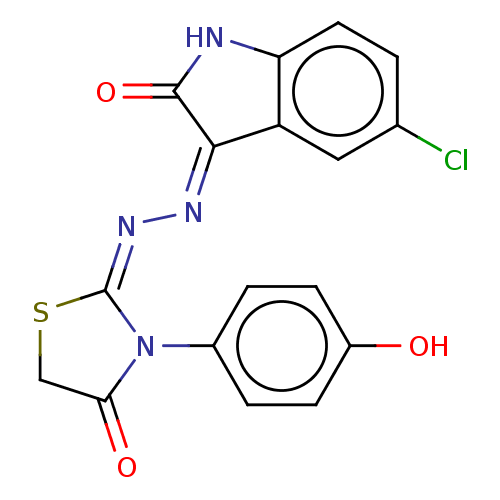
(CHEMBL1761588)Show SMILES Oc1ccc(cc1)N1C(=O)CS\C1=N/N=C1\C(=O)Nc2ccc(Cl)cc12 Show InChI InChI=1S/C17H11ClN4O3S/c18-9-1-6-13-12(7-9)15(16(25)19-13)20-21-17-22(14(24)8-26-17)10-2-4-11(23)5-3-10/h1-7,23H,8H2,(H,19,20,25)/b21-17- | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant TAK1-TAB1 assessed as [33P]gamma-ATP incorporation into substrate histone H1 peptide by filter plate assay |
Bioorg Med Chem Lett 21: 1724-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.077
BindingDB Entry DOI: 10.7270/Q2988794 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347425
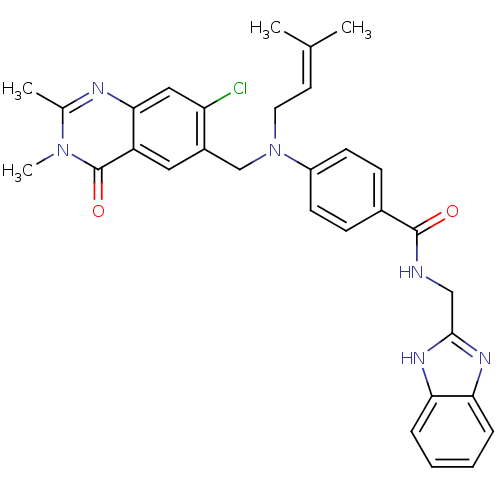
(CHEMBL1801936)Show SMILES CC(C)=CCN(Cc1cc2c(cc1Cl)nc(C)n(C)c2=O)c1ccc(cc1)C(=O)NCc1nc2ccccc2[nH]1 |(1.23,-37.16,;-.11,-36.39,;-1.44,-37.17,;-.11,-34.85,;-1.45,-34.09,;-1.45,-32.55,;-2.79,-31.78,;-4.12,-32.56,;-5.45,-31.79,;-6.78,-32.56,;-6.78,-34.1,;-5.45,-34.87,;-4.12,-34.1,;-2.78,-34.87,;-8.1,-34.86,;-9.43,-34.1,;-10.77,-34.87,;-9.43,-32.56,;-10.77,-31.79,;-8.1,-31.78,;-8.1,-30.24,;-.12,-31.77,;1.21,-32.54,;2.54,-31.77,;2.54,-30.23,;1.19,-29.46,;-.14,-30.24,;3.87,-29.45,;3.86,-27.91,;5.21,-30.21,;6.54,-29.44,;7.87,-30.2,;9.28,-29.57,;10.32,-30.71,;11.85,-30.7,;12.63,-32.02,;11.87,-33.36,;10.33,-33.37,;9.55,-32.04,;8.05,-31.73,)| Show InChI InChI=1S/C31H31ClN6O2/c1-19(2)13-14-38(18-22-15-24-28(16-25(22)32)34-20(3)37(4)31(24)40)23-11-9-21(10-12-23)30(39)33-17-29-35-26-7-5-6-8-27(26)36-29/h5-13,15-16H,14,17-18H2,1-4H3,(H,33,39)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347439
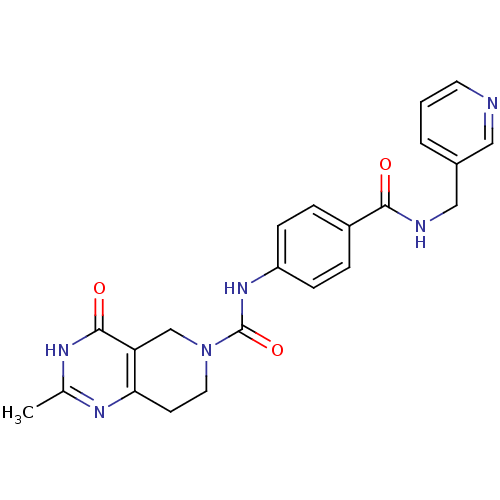
(CHEMBL1801870)Show SMILES Cc1nc2CCN(Cc2c(=O)[nH]1)C(=O)Nc1ccc(cc1)C(=O)NCc1cccnc1 Show InChI InChI=1S/C22H22N6O3/c1-14-25-19-8-10-28(13-18(19)21(30)26-14)22(31)27-17-6-4-16(5-7-17)20(29)24-12-15-3-2-9-23-11-15/h2-7,9,11H,8,10,12-13H2,1H3,(H,24,29)(H,27,31)(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50108067
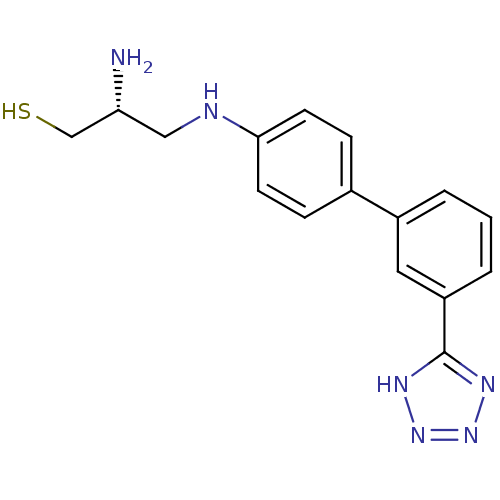
(2-Amino-3-[3'-(5H-tetrazol-5-yl)-biphenyl-4-ylamin...)Show SMILES N[C@@H](CS)CNc1ccc(cc1)-c1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C16H18N6S/c17-14(10-23)9-18-15-6-4-11(5-7-15)12-2-1-3-13(8-12)16-19-21-22-20-16/h1-8,14,18,23H,9-10,17H2,(H,19,20,21,22)/t14-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells |
J Med Chem 45: 177-88 (2001)
BindingDB Entry DOI: 10.7270/Q22F7MQ7 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347444
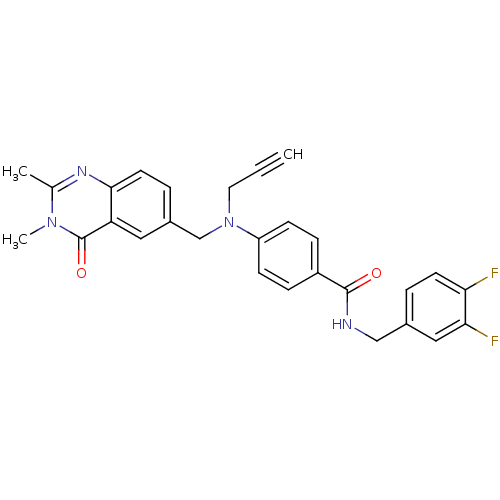
(CHEMBL1801550)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NCc3ccc(F)c(F)c3)cc2c(=O)n1C Show InChI InChI=1S/C28H24F2N4O2/c1-4-13-34(17-20-6-12-26-23(14-20)28(36)33(3)18(2)32-26)22-9-7-21(8-10-22)27(35)31-16-19-5-11-24(29)25(30)15-19/h1,5-12,14-15H,13,16-17H2,2-3H3,(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
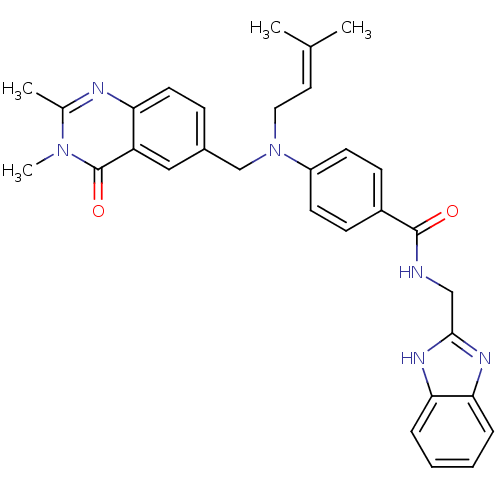
(Homo sapiens (Human)) | BDBM50347414
(CHEMBL1801553)Show SMILES CC(C)=CCN(Cc1ccc2nc(C)n(C)c(=O)c2c1)c1ccc(cc1)C(=O)NCc1nc2ccccc2[nH]1 |(1.79,-27.5,;.45,-26.73,;-.88,-27.5,;.45,-25.19,;-.89,-24.42,;-.9,-22.88,;-2.23,-22.12,;-3.56,-22.89,;-3.56,-24.43,;-4.89,-25.2,;-6.22,-24.43,;-7.55,-25.2,;-8.87,-24.44,;-10.21,-25.21,;-8.87,-22.9,;-10.21,-22.13,;-7.55,-22.12,;-7.55,-20.58,;-6.22,-22.9,;-4.89,-22.13,;.44,-22.11,;1.77,-22.88,;3.1,-22.1,;3.1,-20.56,;1.75,-19.8,;.42,-20.57,;4.43,-19.78,;4.42,-18.24,;5.76,-20.55,;7.1,-19.77,;8.43,-20.54,;9.84,-19.9,;10.88,-21.04,;12.41,-21.03,;13.19,-22.35,;12.43,-23.69,;10.89,-23.7,;10.11,-22.38,;8.61,-22.07,)| Show InChI InChI=1S/C31H32N6O2/c1-20(2)15-16-37(19-22-9-14-26-25(17-22)31(39)36(4)21(3)33-26)24-12-10-23(11-13-24)30(38)32-18-29-34-27-7-5-6-8-28(27)35-29/h5-15,17H,16,18-19H2,1-4H3,(H,32,38)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
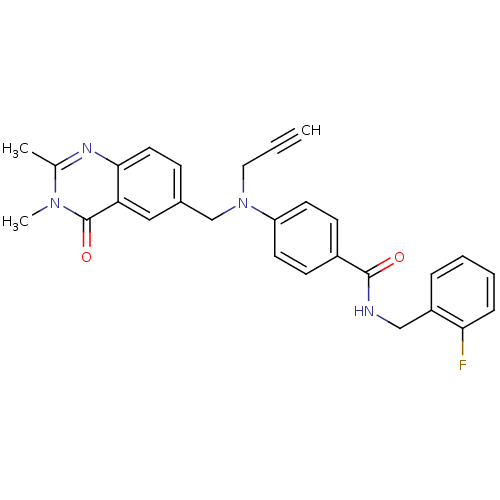
(Homo sapiens (Human)) | BDBM50347420
(CHEMBL1801941)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NCc3ccccc3F)cc2c(=O)n1C Show InChI InChI=1S/C28H25FN4O2/c1-4-15-33(18-20-9-14-26-24(16-20)28(35)32(3)19(2)31-26)23-12-10-21(11-13-23)27(34)30-17-22-7-5-6-8-25(22)29/h1,5-14,16H,15,17-18H2,2-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
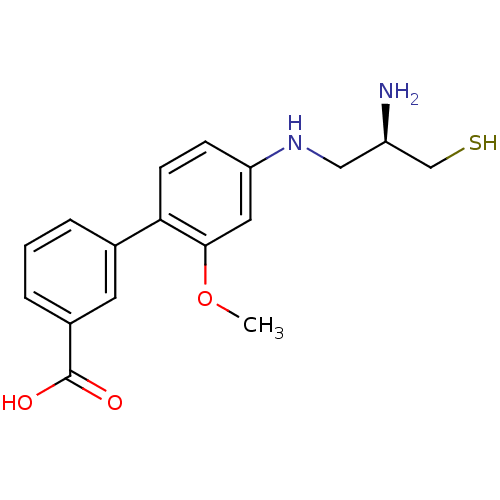
(Homo sapiens (Human)) | BDBM50048961
(4'-((R)-2-Amino-3-mercapto-propylamino)-2'-methoxy...)Show InChI InChI=1S/C17H20N2O3S/c1-22-16-8-14(19-9-13(18)10-23)5-6-15(16)11-3-2-4-12(7-11)17(20)21/h2-8,13,19,23H,9-10,18H2,1H3,(H,20,21)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells |
J Med Chem 45: 177-88 (2001)
BindingDB Entry DOI: 10.7270/Q22F7MQ7 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
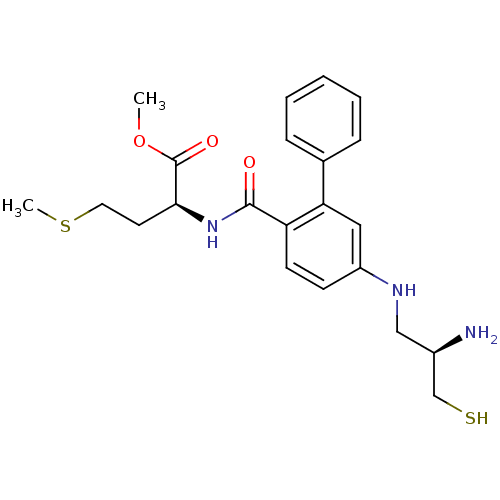
(Homo sapiens (Human)) | BDBM50138764
((S)-2-{[5-((R)-2-Amino-3-mercapto-propylamino)-bip...)Show SMILES COC(=O)[C@H](CCSC)NC(=O)c1ccc(NC[C@@H](N)CS)cc1-c1ccccc1 Show InChI InChI=1S/C22H29N3O3S2/c1-28-22(27)20(10-11-30-2)25-21(26)18-9-8-17(24-13-16(23)14-29)12-19(18)15-6-4-3-5-7-15/h3-9,12,16,20,24,29H,10-11,13-14,23H2,1-2H3,(H,25,26)/t16-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei protein farnesyltransferase |
J Med Chem 47: 432-45 (2004)
Article DOI: 10.1021/jm030236o
BindingDB Entry DOI: 10.7270/Q2TQ60Z4 |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50347443
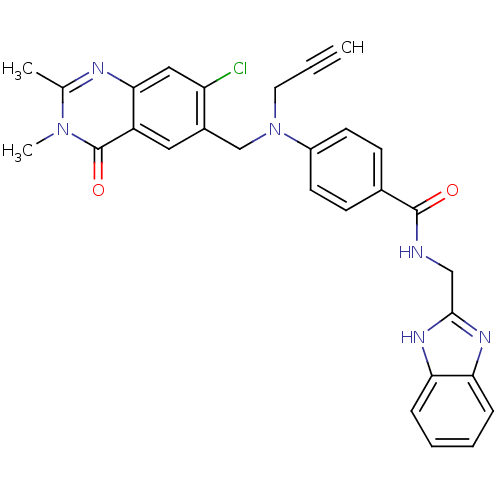
(CHEMBL1801933)Show SMILES Cc1nc2cc(Cl)c(CN(CC#C)c3ccc(cc3)C(=O)NCc3nc4ccccc4[nH]3)cc2c(=O)n1C Show InChI InChI=1S/C29H25ClN6O2/c1-4-13-36(17-20-14-22-26(15-23(20)30)32-18(2)35(3)29(22)38)21-11-9-19(10-12-21)28(37)31-16-27-33-24-7-5-6-8-25(24)34-27/h1,5-12,14-15H,13,16-17H2,2-3H3,(H,31,37)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Myrexis, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nicotinamide phosphoribosyltransferase-catalyzed conversion of nicotinamide to nicotinamide mononucleotide |
J Med Chem 53: 8734-46 (2010)
Article DOI: 10.1021/jm101145b
BindingDB Entry DOI: 10.7270/Q26H4HSP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50097817
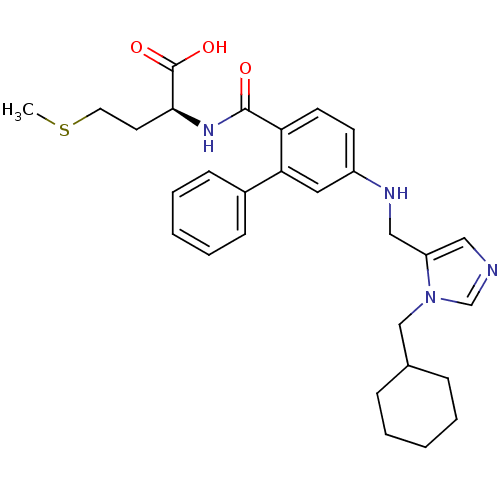
((S)-2-({5-[(3-Cyclohexylmethyl-3H-imidazol-4-ylmet...)Show SMILES CSCC[C@H](NC(=O)c1ccc(NCc2cncn2CC2CCCCC2)cc1-c1ccccc1)C(O)=O Show InChI InChI=1S/C29H36N4O3S/c1-37-15-14-27(29(35)36)32-28(34)25-13-12-23(16-26(25)22-10-6-3-7-11-22)31-18-24-17-30-20-33(24)19-21-8-4-2-5-9-21/h3,6-7,10-13,16-17,20-21,27,31H,2,4-5,8-9,14-15,18-19H2,1H3,(H,32,34)(H,35,36)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of mammalian Farnesyltransferase |
Bioorg Med Chem Lett 11: 761-4 (2001)
BindingDB Entry DOI: 10.7270/Q26W99CH |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50108058
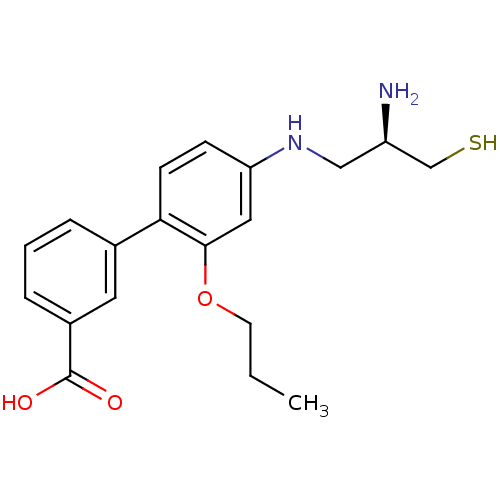
(4'-(2-Amino-3-mercapto-propylamino)-2'-propoxy-bip...)Show SMILES CCCOc1cc(NC[C@@H](N)CS)ccc1-c1cccc(c1)C(O)=O Show InChI InChI=1S/C19H24N2O3S/c1-2-8-24-18-10-16(21-11-15(20)12-25)6-7-17(18)13-4-3-5-14(9-13)19(22)23/h3-7,9-10,15,21,25H,2,8,11-12,20H2,1H3,(H,22,23)/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase from human Burkitt lymphoma (Daudi) cells |
J Med Chem 45: 177-88 (2001)
BindingDB Entry DOI: 10.7270/Q22F7MQ7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data