 Found 46 hits with Last Name = 'long' and Initial = 'bj'
Found 46 hits with Last Name = 'long' and Initial = 'bj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063477

((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063476

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063475
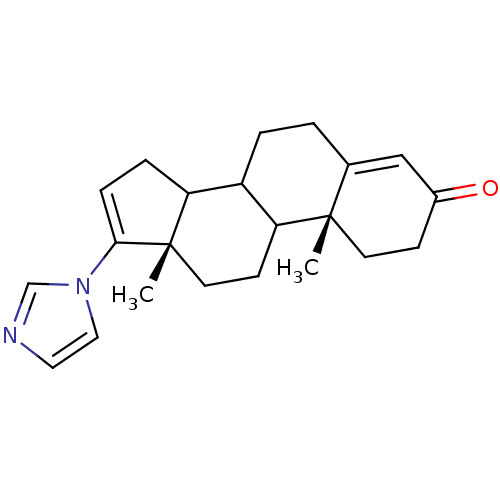
((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063479
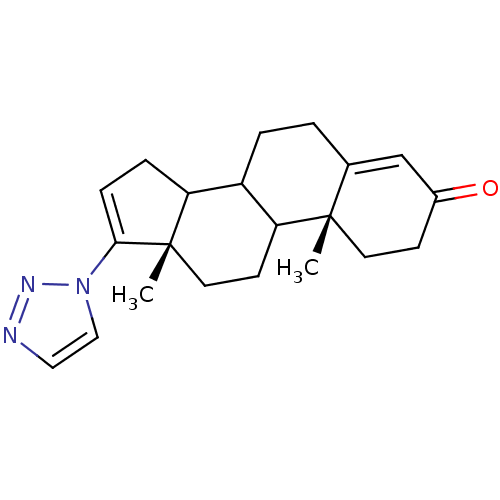
((10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063480

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063478
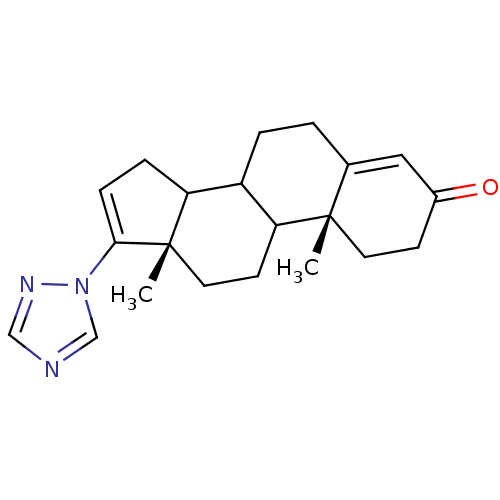
((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Binding affinity for Cytochrome P450 17A1 (17-alpha-hydroxypregnenolone Km=560 nM) |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604556
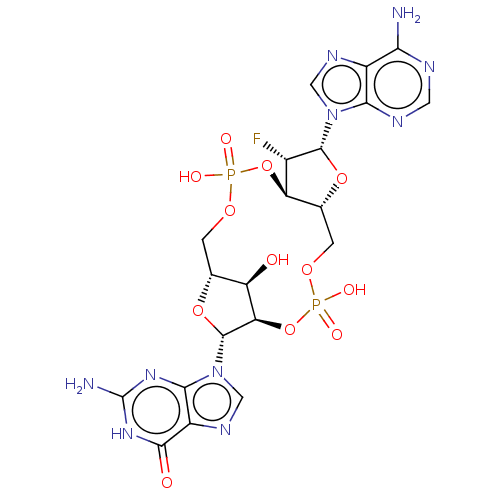
(CHEMBL5175703)Show SMILES N.N.[H][C@@]12COP(O)(=O)O[C@@]3([H])[C@H](F)[C@@H](O[C@]3([H])COP(O)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509399
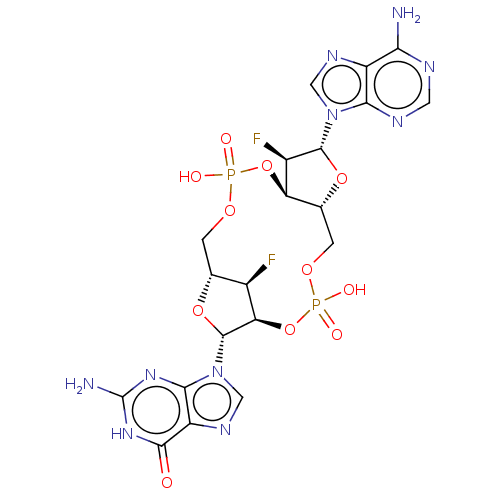
(CHEMBL4468010)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@@H](COP(O)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@@H]3F)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H22F2N10O11P2/c21-8-6-1-38-44(34,35)42-12-7(41-18(9(12)22)31-4-27-10-14(23)25-3-26-15(10)31)2-39-45(36,37)43-13(8)19(40-6)32-5-28-11-16(32)29-20(24)30-17(11)33/h3-9,12-13,18-19H,1-2H2,(H,34,35)(H,36,37)(H2,23,25,26)(H3,24,29,30,33)/t6-,7-,8-,9-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509421
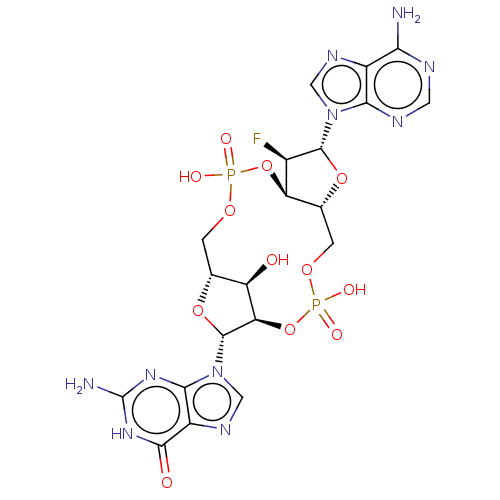
(CHEMBL4577528)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@@H](COP(O)(=O)O[C@@H]1[C@@H]2O)O[C@H]([C@@H]3F)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C20H23FN10O12P2/c21-8-12-7(41-18(8)30-4-26-9-14(22)24-3-25-15(9)30)2-39-45(36,37)43-13-11(32)6(1-38-44(34,35)42-12)40-19(13)31-5-27-10-16(31)28-20(23)29-17(10)33/h3-8,11-13,18-19,32H,1-2H2,(H,34,35)(H,36,37)(H2,22,24,25)(H3,23,28,29,33)/t6-,7-,8-,11-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stimulator of interferon genes protein
(Human) | BDBM573971
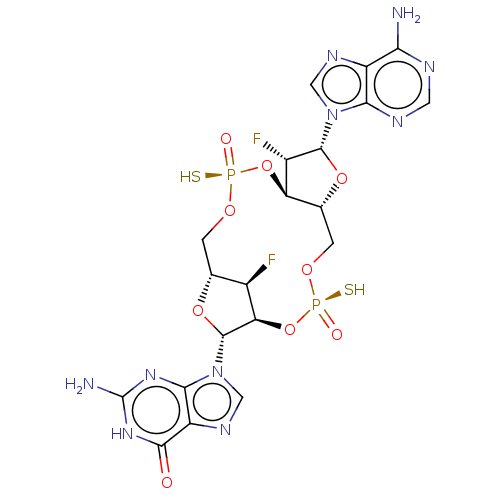
(US11453697, Example 247)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2CO[P@](S)(=O)O[C@@H]3[C@@H](CO[P@](S)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@H]3F)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509408
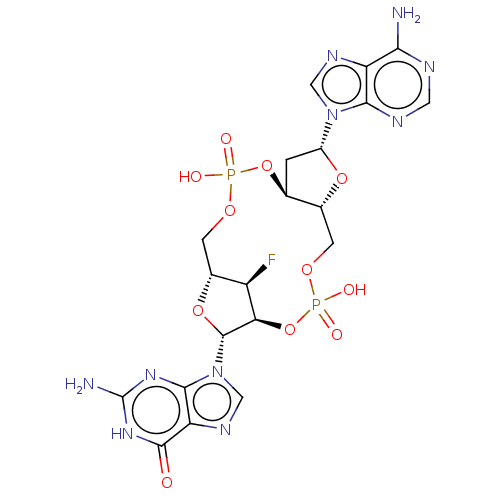
(CHEMBL4459563)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@@H]1[C@@H]2F)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H23FN10O11P2/c21-11-9-3-38-43(33,34)41-7-1-10(30-5-26-12-15(22)24-4-25-16(12)30)39-8(7)2-37-44(35,36)42-14(11)19(40-9)31-6-27-13-17(31)28-20(23)29-18(13)32/h4-11,14,19H,1-3H2,(H,33,34)(H,35,36)(H2,22,24,25)(H3,23,28,29,32)/t7-,8+,9+,10+,11+,14+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509422
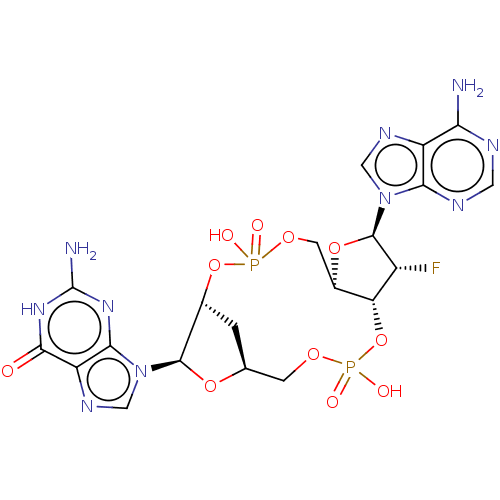
(CHEMBL4476079)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H]2C[C@H]1OP(O)(=O)OC[C@H]1O[C@H]([C@H](F)[C@@H]1OP(O)(=O)OC2)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H23FN10O11P2/c21-10-13-9(40-19(10)30-5-26-11-14(22)24-4-25-15(11)30)3-38-43(33,34)41-8-1-7(2-37-44(35,36)42-13)39-18(8)31-6-27-12-16(31)28-20(23)29-17(12)32/h4-10,13,18-19H,1-3H2,(H,33,34)(H,35,36)(H2,22,24,25)(H3,23,28,29,32)/t7-,8+,9+,10+,13+,18+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM573856
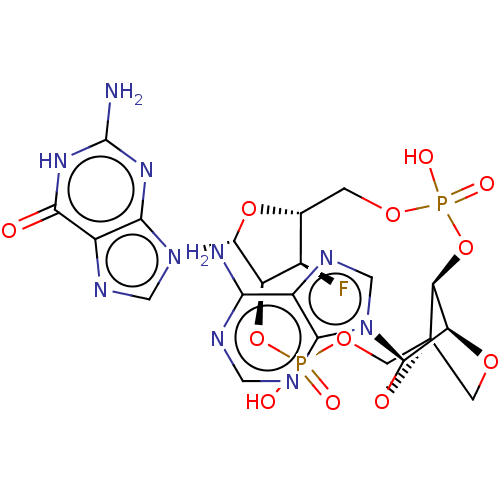
(US11453697, Example 20)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@H]3[C@H]4OC[C@]3(COP(O)(=O)O[C@@H]1[C@@H]2F)O[C@H]4n1cnc2c(N)ncnc12 |r,TLB:19:20:35.34:23.22| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509431
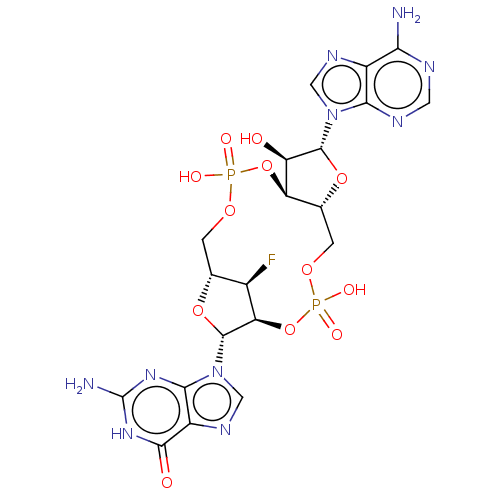
(CHEMBL4589856)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@@H]3[C@@H](COP(O)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@@H]3O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H23FN10O12P2/c21-8-6-1-38-44(34,35)42-12-7(41-18(11(12)32)30-4-26-9-14(22)24-3-25-15(9)30)2-39-45(36,37)43-13(8)19(40-6)31-5-27-10-16(31)28-20(23)29-17(10)33/h3-8,11-13,18-19,32H,1-2H2,(H,34,35)(H,36,37)(H2,22,24,25)(H3,23,28,29,33)/t6-,7-,8-,11-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604561
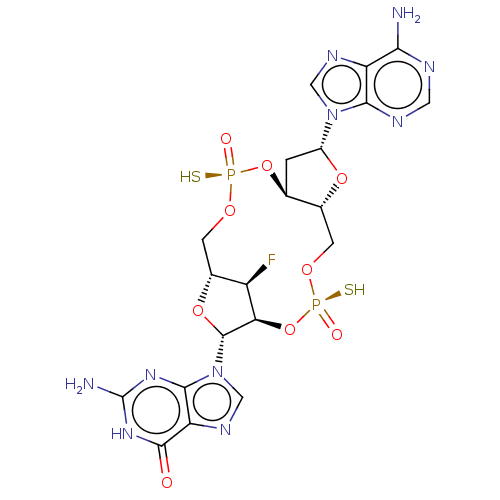
(CHEMBL5197904)Show SMILES N.N.[H][C@@]12CO[P@](S)(=O)O[C@@]3([H])C[C@@H](O[C@]3([H])CO[P@](S)(=O)O[C@]([H])([C@@H]1F)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50506263
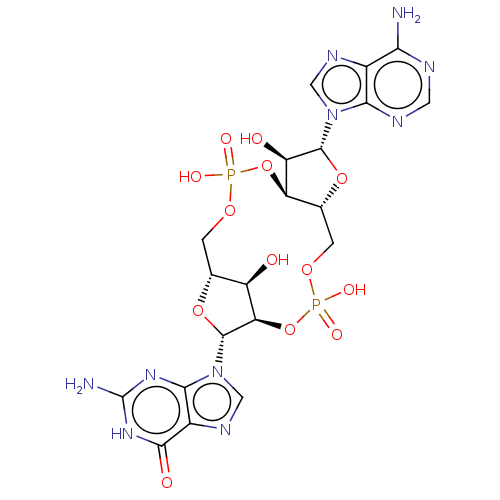
(CHEMBL4465054 | US11401295, Compound 2',3'-cGAMP)Show SMILES [H][C@]12COP(O)(=O)O[C@@]3([H])[C@@H](O)[C@@H](O[C@]3([H])COP(O)(=O)O[C@@]([H])([C@@H](O1)n1cnc3c1nc(N)[nH]c3=O)[C@@H]2O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O13P2/c21-14-8-15(24-3-23-14)29(4-25-8)18-11(32)12-7(41-18)2-39-45(36,37)43-13-10(31)6(1-38-44(34,35)42-12)40-19(13)30-5-26-9-16(30)27-20(22)28-17(9)33/h3-7,10-13,18-19,31-32H,1-2H2,(H,34,35)(H,36,37)(H2,21,23,24)(H3,22,27,28,33)/t6-,7-,10-,11-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604563
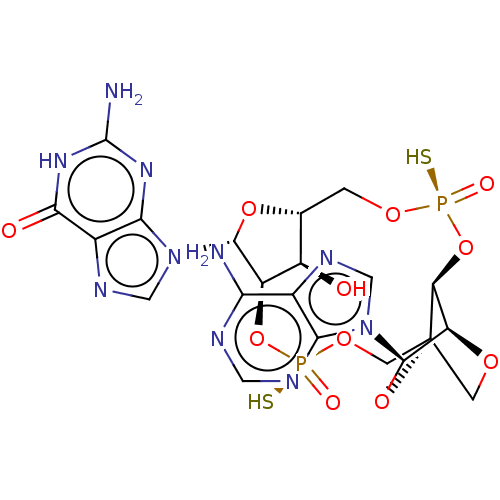
(CHEMBL5201164)Show SMILES N.N.[H][C@@]12CO[P@](S)(=O)O[C@@]3([H])[C@@]4([H])OC[C@]3(CO[P@](S)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)O[C@H]4n1cnc2c(N)ncnc12 |r,TLB:9:10:40.41:14.15| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604560
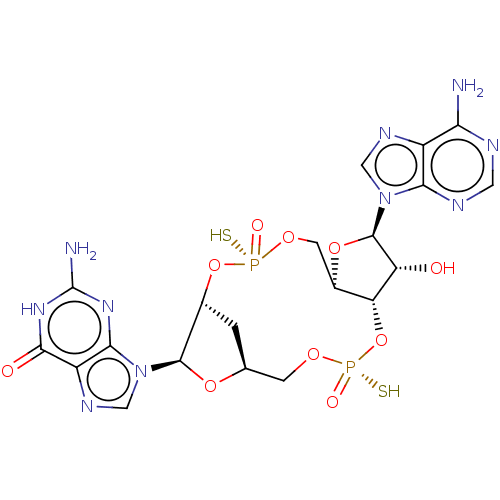
(CHEMBL5180005)Show SMILES N.N.[H][C@]12C[C@@]([H])(O[P@@](S)(=O)OC[C@@]3([H])O[C@H]([C@H](O)[C@]3([H])O[P@@](S)(=O)OC1)n1cnc3c(N)ncnc13)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604555
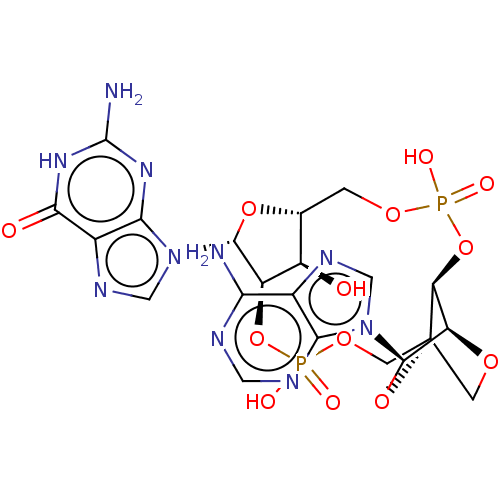
(CHEMBL5181746)Show SMILES N.N.[H][C@@]12COP(O)(=O)O[C@@]3([H])[C@@]4([H])OC[C@]3(COP(O)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)O[C@H]4n1cnc2c(N)ncnc12 |r,TLB:9:10:40.41:14.15| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604562
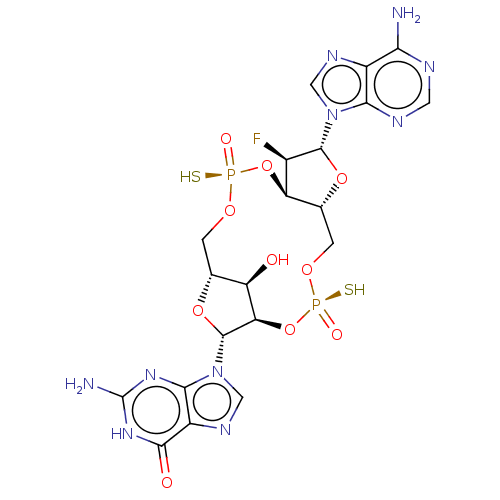
(CHEMBL5185127)Show SMILES N.N.[H][C@@]12CO[P@](S)(=O)O[C@@]3([H])[C@@H](F)[C@@H](O[C@]3([H])CO[P@](S)(=O)O[C@]([H])([C@@H]1O)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509412
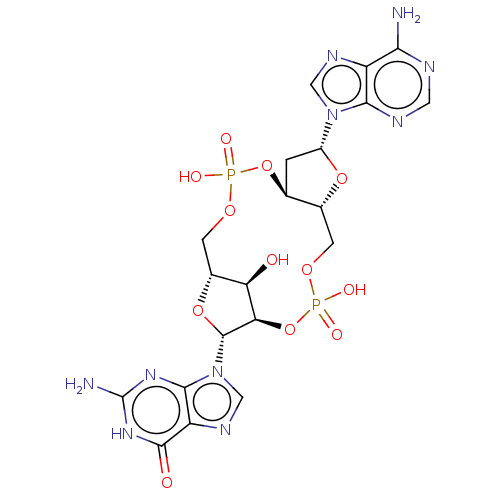
(CHEMBL4474321 | US11453697, Example 1)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2COP(O)(=O)O[C@H]3C[C@@H](O[C@@H]3COP(O)(=O)O[C@@H]1[C@@H]2O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O12P2/c21-15-11-16(24-4-23-15)29(5-25-11)10-1-7-8(39-10)2-37-44(35,36)42-14-13(31)9(3-38-43(33,34)41-7)40-19(14)30-6-26-12-17(30)27-20(22)28-18(12)32/h4-10,13-14,19,31H,1-3H2,(H,33,34)(H,35,36)(H2,21,23,24)(H3,22,27,28,32)/t7-,8+,9+,10+,13+,14+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604559
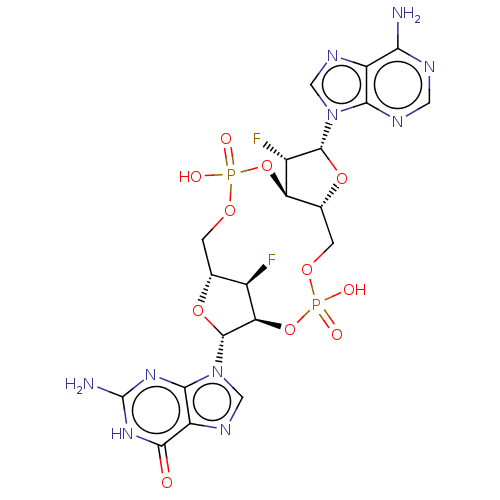
(CHEMBL5174023)Show SMILES N.N.[H][C@@]12COP(O)(=O)O[C@@]3([H])[C@H](F)[C@@H](O[C@]3([H])COP(O)(=O)O[C@]([H])([C@@H]1F)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604557
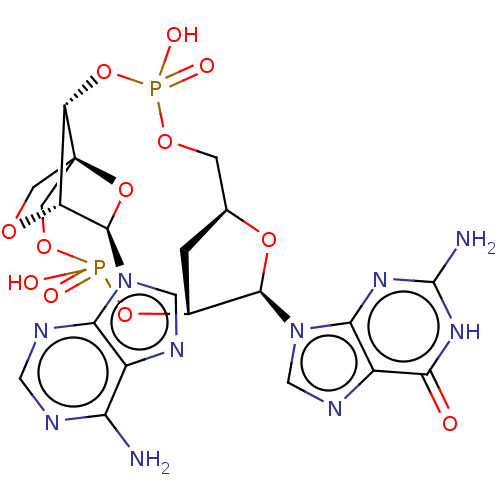
(CHEMBL5191008)Show SMILES N.N.[H][C@]12C[C@@]([H])(OP(O)(=O)OC[C@@]34CO[C@@]([H])([C@@H](O3)n3cnc5c(N)ncnc35)[C@]4([H])OP(O)(=O)OC1)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O |r,TLB:32:30:14.15:18.19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063475

((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063475

((10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-1,2,6,7,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:8| Show InChI InChI=1S/C22H28N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h6,11-14,17-19H,3-5,7-10H2,1-2H3/t17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063477

((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17?,18?,19?,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509430
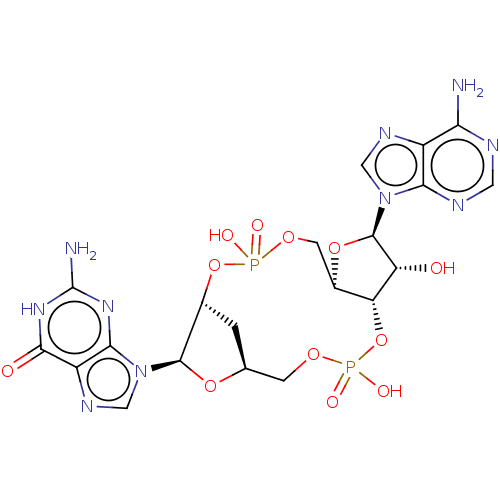
(CHEMBL4570468)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H]2C[C@H]1OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(=O)OC2)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O12P2/c21-14-10-15(24-4-23-14)29(5-25-10)19-12(31)13-9(40-19)3-38-43(33,34)41-8-1-7(2-37-44(35,36)42-13)39-18(8)30-6-26-11-16(30)27-20(22)28-17(11)32/h4-9,12-13,18-19,31H,1-3H2,(H,33,34)(H,35,36)(H2,21,23,24)(H3,22,27,28,32)/t7-,8+,9+,12+,13+,18+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063479

((10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063477

((3S,10R,13S)-17-Imidazol-1-yl-10,13-dimethyl-2,3,4...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnc1 |c:21,t:7| Show InChI InChI=1S/C22H30N2O/c1-21-9-7-16(25)13-15(21)3-4-17-18-5-6-20(24-12-11-23-14-24)22(18,2)10-8-19(17)21/h3,6,11-12,14,16-19,25H,4-5,7-10,13H2,1-2H3/t16-,17?,18?,19?,21-,22-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50509425
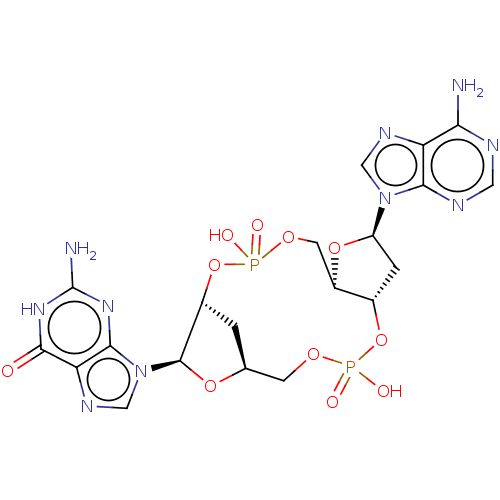
(CHEMBL4566174 | US11453697, Example 27)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H]2C[C@H]1OP(O)(=O)OC[C@H]1O[C@H](C[C@@H]1OP(O)(=O)OC2)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O11P2/c21-15-13-16(24-5-23-15)29(6-25-13)12-2-9-11(39-12)4-37-43(34,35)41-10-1-8(3-36-42(32,33)40-9)38-19(10)30-7-26-14-17(30)27-20(22)28-18(14)31/h5-12,19H,1-4H2,(H,32,33)(H,34,35)(H2,21,23,24)(H3,22,27,28,31)/t8-,9-,10+,11+,12+,19+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063476

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063478

((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063476

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h3,6,11-12,15-18,25H,4-5,7-10,13H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50604558

(CHEMBL5205731)Show SMILES N.N.[H][C@]12C[C@@]([H])(OP(O)(=O)OC[C@@]3([H])O[C@H]([C@@H](F)[C@]3([H])OP(O)(=O)OC1)n1cnc3c(N)ncnc13)[C@@H](O2)n1cnc2c1nc(N)[nH]c2=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063479

((10R,13S)-10,13-Dimethyl-17-[1,2,3]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1ccnn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)13-14(20)3-4-16-17-5-6-19(24-12-11-22-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50063480

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50582513
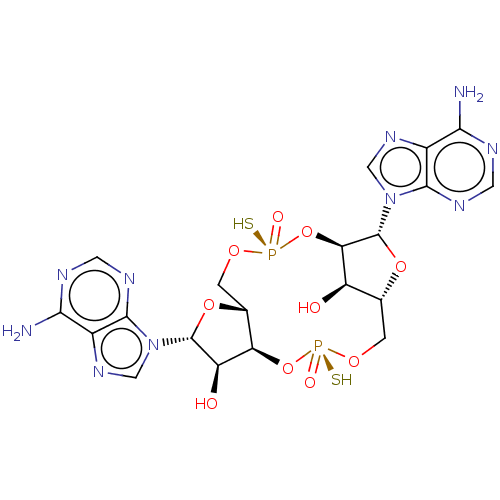
(CHEMBL5093161)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@@H]2CO[P@](S)(=O)O[C@@H]3[C@H](O)[C@@H](CO[P@](S)(=O)O[C@H]2[C@H]1O)O[C@H]3n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063478

((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...)Show SMILES C[C@]12CCC3C(CCC4=CC(=O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:8| Show InChI InChI=1S/C21H27N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h6,11-13,16-18H,3-5,7-10H2,1-2H3/t16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50063480

((3S,10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl...)Show SMILES C[C@]12CCC3C(CC=C4C[C@@H](O)CC[C@]34C)C1CC=C2n1cncn1 |c:21,t:7| Show InChI InChI=1S/C21H29N3O/c1-20-9-7-15(25)11-14(20)3-4-16-17-5-6-19(24-13-22-12-23-24)21(17,2)10-8-18(16)20/h3,6,12-13,15-18,25H,4-5,7-11H2,1-2H3/t15-,16?,17?,18?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of human testicular microsomal Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| Assay Description
Inhibition of rat Cytochrome P450 17A1 |
J Med Chem 41: 902-12 (1998)
Article DOI: 10.1021/jm970568r
BindingDB Entry DOI: 10.7270/Q2GH9H2N |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Mus musculus) | BDBM50506263

(CHEMBL4465054 | US11401295, Compound 2',3'-cGAMP)Show SMILES [H][C@]12COP(O)(=O)O[C@@]3([H])[C@@H](O)[C@@H](O[C@]3([H])COP(O)(=O)O[C@@]([H])([C@@H](O1)n1cnc3c1nc(N)[nH]c3=O)[C@@H]2O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O13P2/c21-14-8-15(24-3-23-14)29(4-25-8)18-11(32)12-7(41-18)2-39-45(36,37)43-13-10(31)6(1-38-44(34,35)42-12)40-19(13)30-5-26-9-16(30)27-20(22)28-17(9)33/h3-7,10-13,18-19,31-32H,1-2H2,(H,34,35)(H,36,37)(H2,21,23,24)(H3,22,27,28,33)/t6-,7-,10-,11-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | <0.0100 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM573971

(US11453697, Example 247)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2CO[P@](S)(=O)O[C@@H]3[C@@H](CO[P@](S)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@H]3F)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Human) | BDBM50506263

(CHEMBL4465054 | US11401295, Compound 2',3'-cGAMP)Show SMILES [H][C@]12COP(O)(=O)O[C@@]3([H])[C@@H](O)[C@@H](O[C@]3([H])COP(O)(=O)O[C@@]([H])([C@@H](O1)n1cnc3c1nc(N)[nH]c3=O)[C@@H]2O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C20H24N10O13P2/c21-14-8-15(24-3-23-14)29(4-25-8)18-11(32)12-7(41-18)2-39-45(36,37)43-13-10(31)6(1-38-44(34,35)42-12)40-19(13)30-5-26-9-16(30)27-20(22)28-17(9)33/h3-7,10-13,18-19,31-32H,1-2H2,(H,34,35)(H,36,37)(H2,21,23,24)(H3,22,27,28,33)/t6-,7-,10-,11-,12-,13-,18-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |
Stimulator of interferon genes protein
(Mus musculus) | BDBM573971

(US11453697, Example 247)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@@H]2CO[P@](S)(=O)O[C@@H]3[C@@H](CO[P@](S)(=O)O[C@@H]1[C@@H]2F)O[C@H]([C@H]3F)n1cnc2c(N)ncnc12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02197
BindingDB Entry DOI: 10.7270/Q2028WM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data