

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947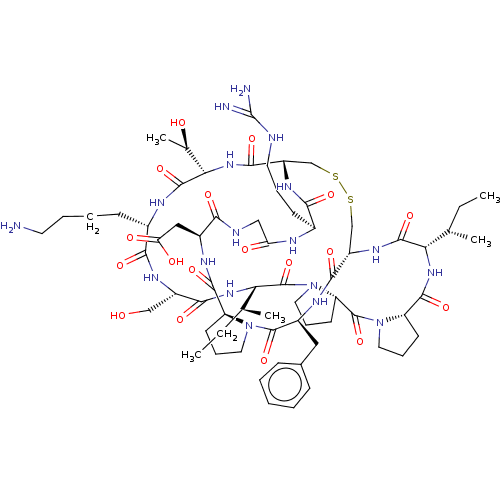 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin type-3 using L-BAPNA as substrate preincubated for 5 mins followed by substrate addition measured over 60 mins | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using N-t-Boc Gln-Ala-Arg-AMC as substrate preincubated with enzyme followed by substrate addition by Dixon plot an... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using BAPNA as substrate preincubated for 15 mins followed by substrate addition measured over 60 mins by Dixon plo... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545388 (CHEMBL4647561) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545392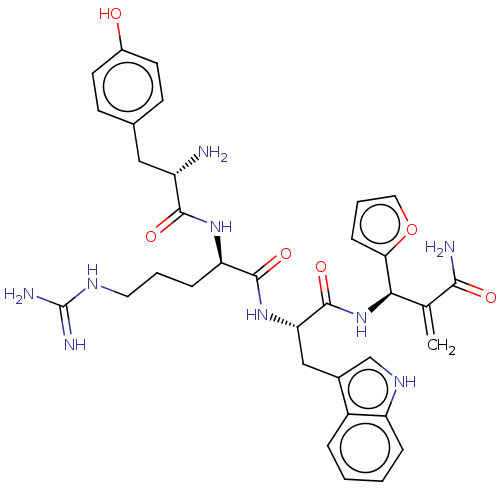 (CHEMBL4642564) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50233907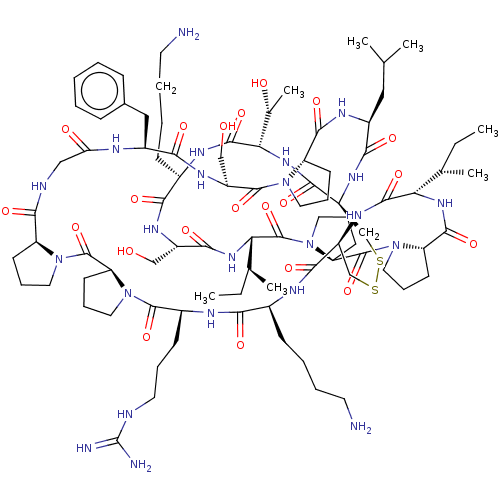 (CHEMBL4102547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin using BAPNA as substrate preincubated for 15 mins followed by substrate addition measured over 60 mins by Dixon plo... | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545394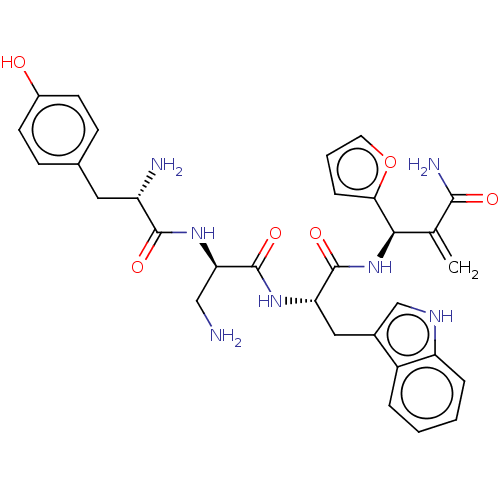 (CHEMBL4640310) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545390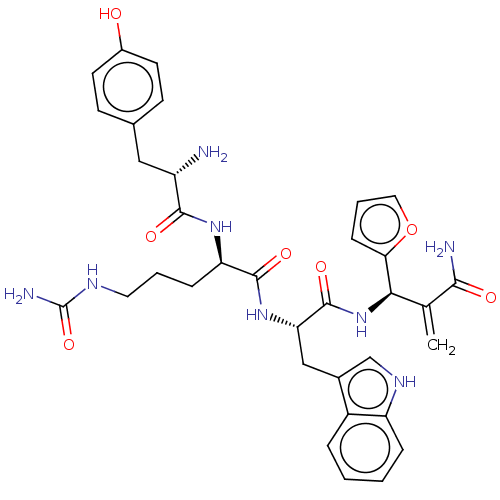 (CHEMBL4633024) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545386 (CHEMBL4644694) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545389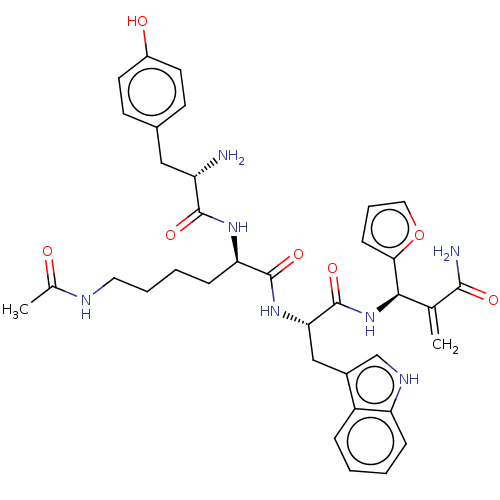 (CHEMBL4646736) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50124947 (CHEMBL453539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University Curated by ChEMBL | Assay Description Inhibition of bovine beta-trypsin | J Med Chem 60: 504-510 (2017) Article DOI: 10.1021/acs.jmedchem.6b01011 BindingDB Entry DOI: 10.7270/Q2RJ4MRS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50095155 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50139013 ((S)-1-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545387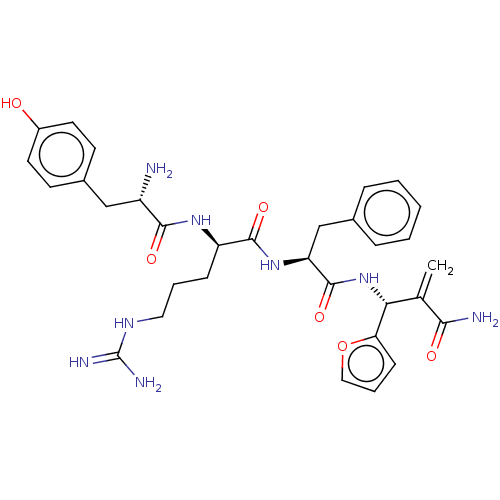 (CHEMBL4645094) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545385 (CHEMBL4635430) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545395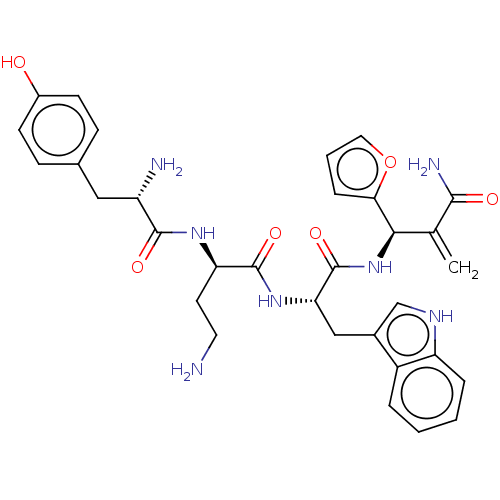 (CHEMBL4638662) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545391 (CHEMBL4638811) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545398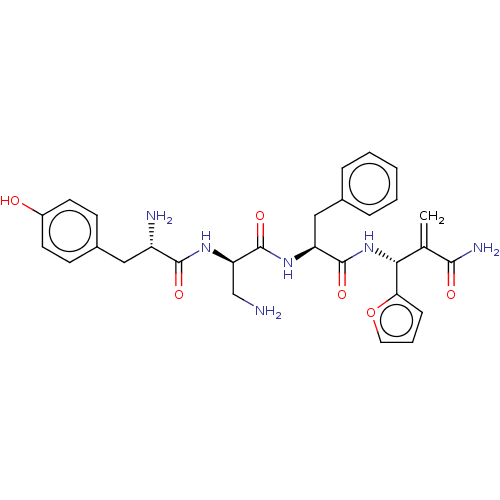 (CHEMBL4642698) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579459 (CHEMBL4863322) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579460 (CHEMBL4857707) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579456 (CHEMBL4863017) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579458 (CHEMBL4862554) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545397 (CHEMBL4635184) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579455 (CHEMBL4873816) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579457 (CHEMBL4846481) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545396 (CHEMBL4638691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545393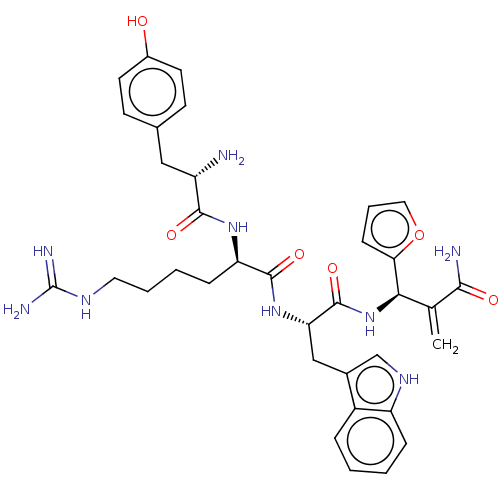 (CHEMBL4634908) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 405 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545396 (CHEMBL4638691) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DAMGO from Wistar rat mu opioid receptor incubated for 1 hr by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579461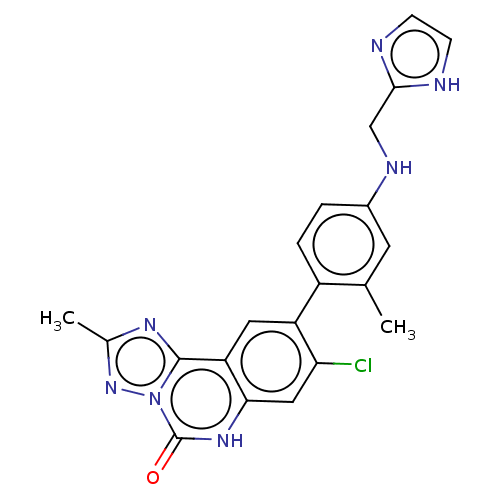 (CHEMBL4854153) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579453 (CHEMBL4875904) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579455 (CHEMBL4873816) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579456 (CHEMBL4863017) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545387 (CHEMBL4645094) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545392 (CHEMBL4642564) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579454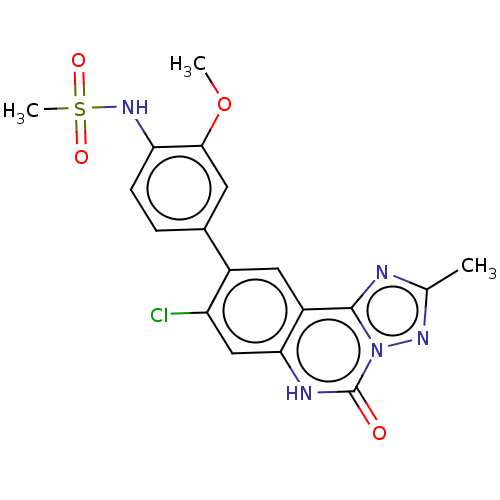 (CHEMBL4868118) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579453 (CHEMBL4875904) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579452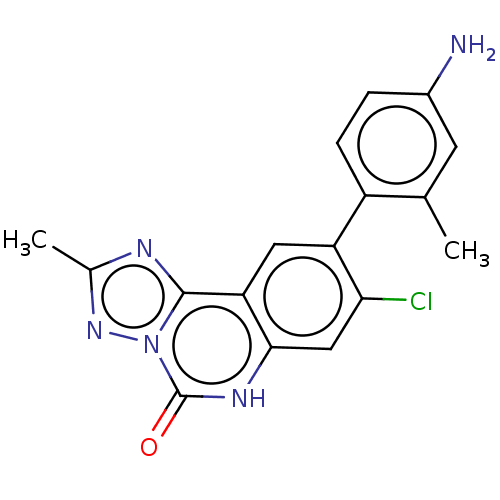 (CHEMBL4846039) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579447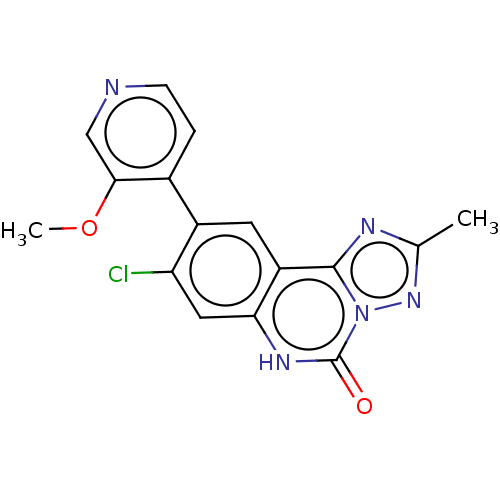 (CHEMBL4874818) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579445 (CHEMBL4859588) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579448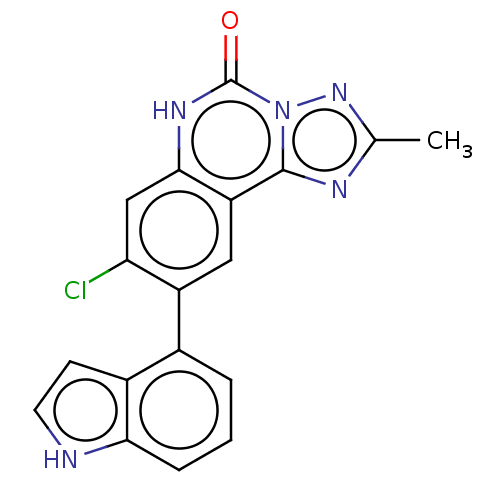 (CHEMBL4851756) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579446 (CHEMBL4860976) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579449 (CHEMBL4872496) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579450 (CHEMBL4876721) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579451 (CHEMBL4864303) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545391 (CHEMBL4638811) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545389 (CHEMBL4646736) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579435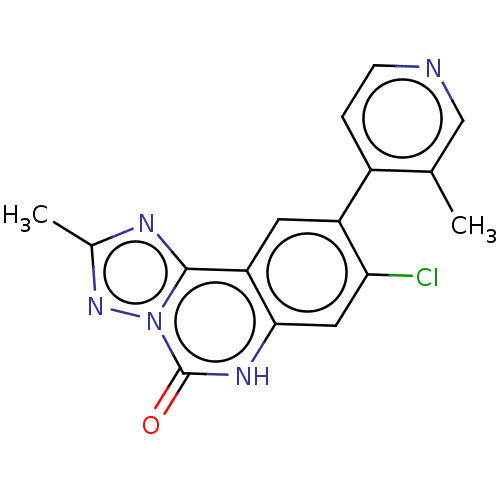 (CHEMBL4863477) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545390 (CHEMBL4633024) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545393 (CHEMBL4634908) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50545394 (CHEMBL4640310) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lanzhou University Curated by ChEMBL | Assay Description Displacement of [3H]-DPDPE from Wistar rat delta opioid receptor incubated for 3 hrs by liquid scintillation counting method | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2020.115438 BindingDB Entry DOI: 10.7270/Q2F47SQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 220 total ) | Next | Last >> |