

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Mixed inhibition of Electrophorus electricus acetylcholinesterase using acetylcholine as substrate by Ellman's method | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401238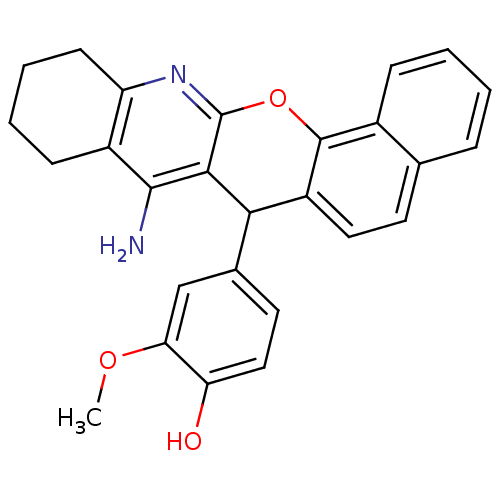 (CHEMBL2206895) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340111 (CHEMBL1762829 | rac-14-(3'-Methoxyphenyl)-10,11,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340110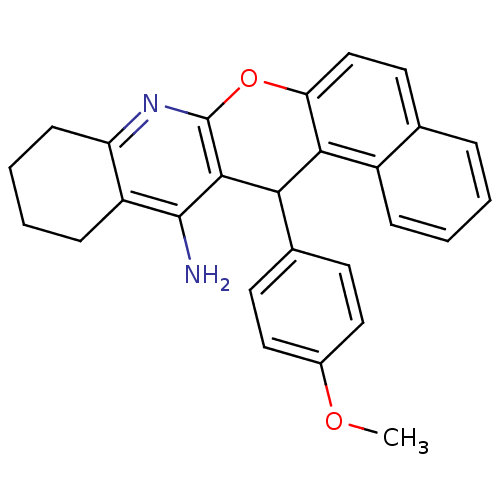 (CHEMBL1762828 | rac-14-(4'-Methoxyphenyl)-10,11,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340112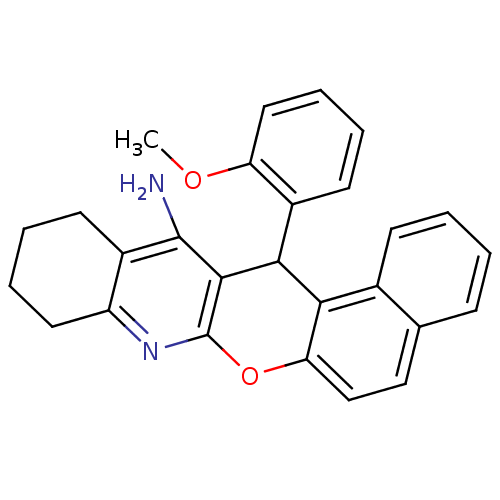 (CHEMBL1762830 | rac-14-(2'-Methoxyphenyl)-10,11,12...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340115 (CHEMBL1762833 | rac-14-(3',4'-Dimethoxyphenyl)-10,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340116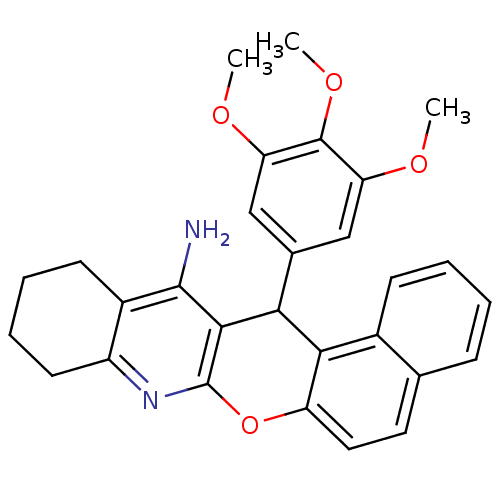 (CHEMBL1762834 | rac-14-(3',4',5'-Trimethoxyphenyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340114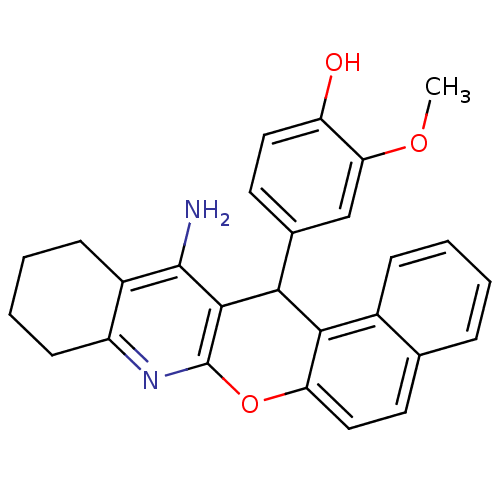 (CHEMBL1762832 | rac-4-[13-Amino-10,11,12,14-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340108 (CHEMBL1762826 | rac-14-(4'-Methylphenyl)-10,11,12,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340113 (CHEMBL1762831 | rac-4-[12-Amino-9,10,11,13-tetrahy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50340117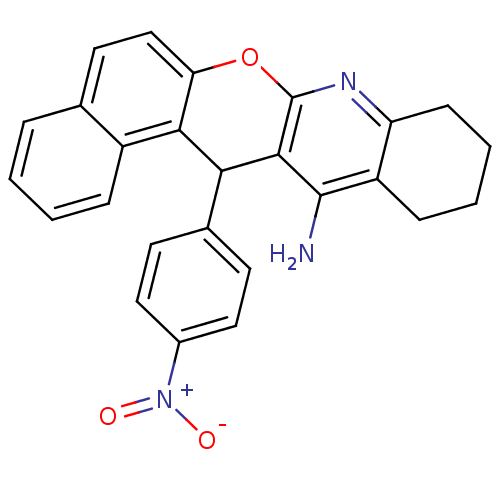 (CHEMBL1762835 | rac-14-(4'-Nitrophenyl)-10,11,12,1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401247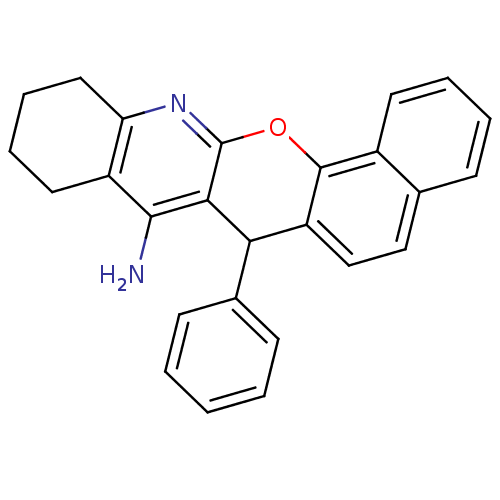 (CHEMBL2206891) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401238 (CHEMBL2206895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401243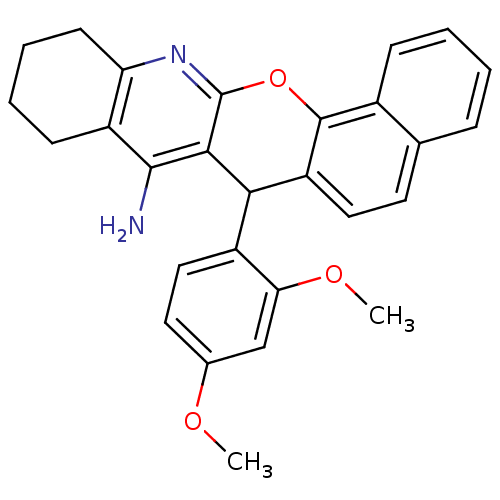 (CHEMBL2206896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401246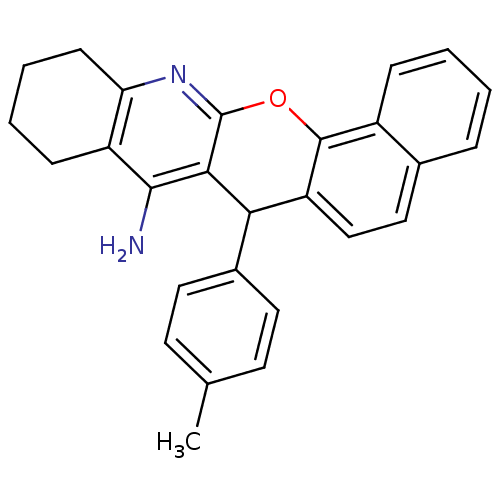 (CHEMBL2206892) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50401248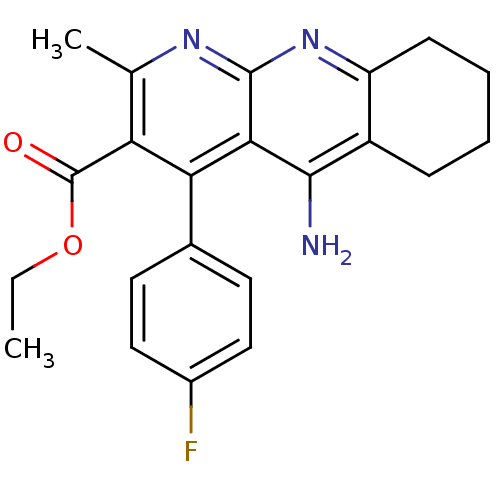 (CHEMBL252380) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of electric eel AChE | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50241346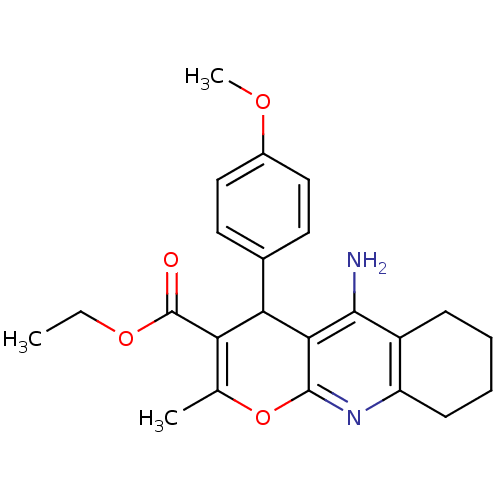 (10-Amino-4-(4-methoxy-phenyl)-2-methyl-5,6,7,8-tet...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 868 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus acetylcholinesterase preincubated with compound for 10 mins using acetylcholine iodide as substrate after 15 m... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401242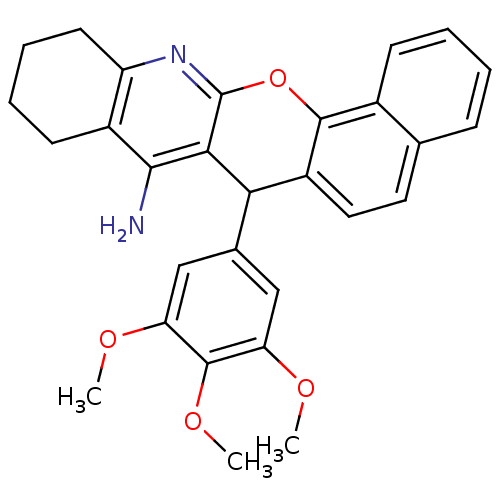 (CHEMBL2206897) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401240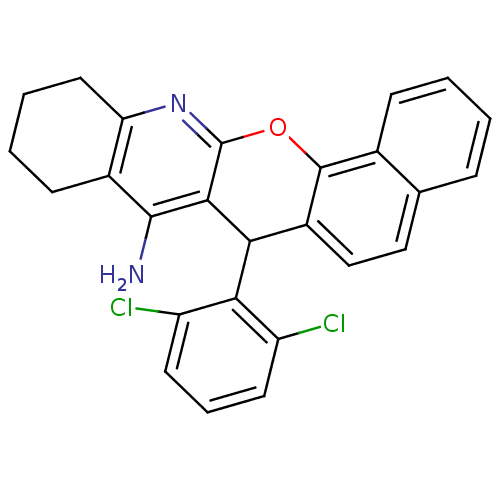 (CHEMBL2206899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401244 (CHEMBL2206894) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401239 (CHEMBL2206900) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401241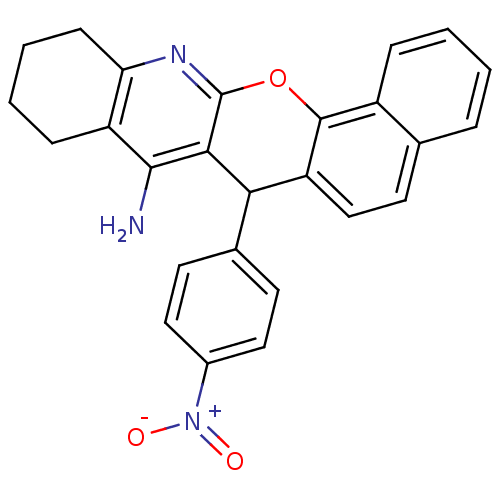 (CHEMBL2206898) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50401245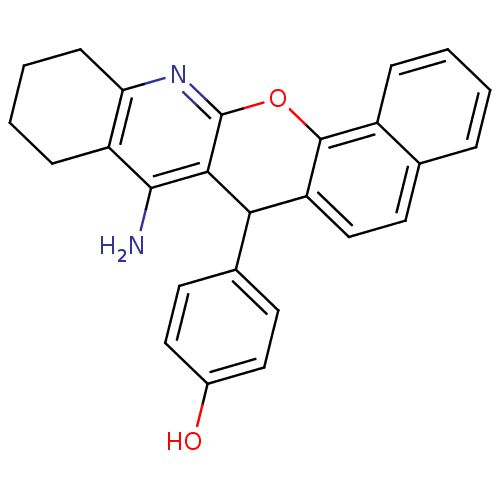 (CHEMBL2206893) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by Ellm... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401244 (CHEMBL2206894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401245 (CHEMBL2206893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401246 (CHEMBL2206892) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401247 (CHEMBL2206891) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401238 (CHEMBL2206895) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401243 (CHEMBL2206896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401242 (CHEMBL2206897) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401241 (CHEMBL2206898) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401240 (CHEMBL2206899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50401239 (CHEMBL2206900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of human BuChE using butyrylthiocholine iodide as substrate incubated for 10 mins prior to substrate addition measured after 15 mins by El... | Eur J Med Chem 54: 750-63 (2012) Article DOI: 10.1016/j.ejmech.2012.06.038 BindingDB Entry DOI: 10.7270/Q2R78GCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340108 (CHEMBL1762826 | rac-14-(4'-Methylphenyl)-10,11,12,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340117 (CHEMBL1762835 | rac-14-(4'-Nitrophenyl)-10,11,12,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340116 (CHEMBL1762834 | rac-14-(3',4',5'-Trimethoxyphenyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340115 (CHEMBL1762833 | rac-14-(3',4'-Dimethoxyphenyl)-10,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340114 (CHEMBL1762832 | rac-4-[13-Amino-10,11,12,14-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340113 (CHEMBL1762831 | rac-4-[12-Amino-9,10,11,13-tetrahy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340112 (CHEMBL1762830 | rac-14-(2'-Methoxyphenyl)-10,11,12...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340109 (CHEMBL1762827 | rac-4-[(13-Amino-10,11,12,14-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340110 (CHEMBL1762828 | rac-14-(4'-Methoxyphenyl)-10,11,12...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50340111 (CHEMBL1762829 | rac-14-(3'-Methoxyphenyl)-10,11,12...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Sfax Curated by ChEMBL | Assay Description Inhibition of horse serum butrylcholinesterase preincubated with compound for 10 mins using butrylcholine iodide as substrate after 15 mins by spectr... | Bioorg Med Chem Lett 21: 2384-8 (2011) Article DOI: 10.1016/j.bmcl.2011.02.094 BindingDB Entry DOI: 10.7270/Q2D79BQQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||