 Found 94 hits with Last Name = 'magata' and Initial = 't'
Found 94 hits with Last Name = 'magata' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50214615
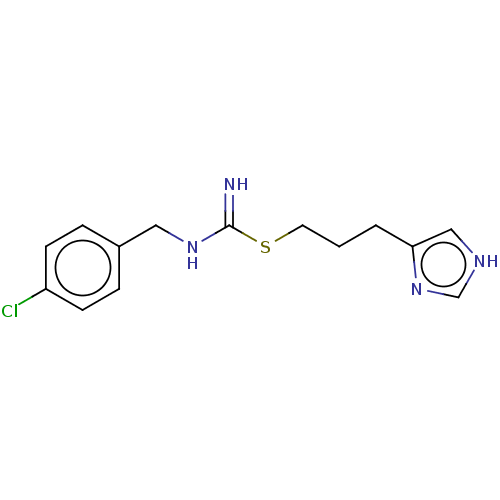
(CHEBI:64177 | Clobenpropit)Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494136
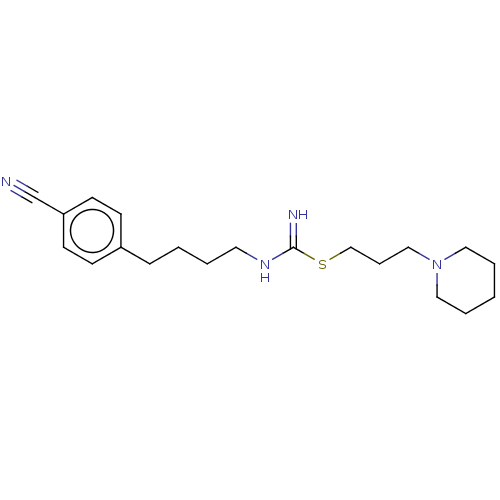
(CHEMBL2441945)Show InChI InChI=1S/C20H30N4S/c21-17-19-10-8-18(9-11-19)7-2-3-12-23-20(22)25-16-6-15-24-13-4-1-5-14-24/h8-11H,1-7,12-16H2,(H2,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494132

(CHEMBL2441941)Show InChI InChI=1S/C18H28ClN3S/c19-17-9-7-16(8-10-17)6-4-11-21-18(20)23-15-5-14-22-12-2-1-3-13-22/h7-10H,1-6,11-15H2,(H2,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494138

(CHEMBL2441946)Show InChI InChI=1S/C18H36N4S/c19-18(23-17-9-16-22-13-6-2-7-14-22)20-10-3-8-15-21-11-4-1-5-12-21/h1-17H2,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494137
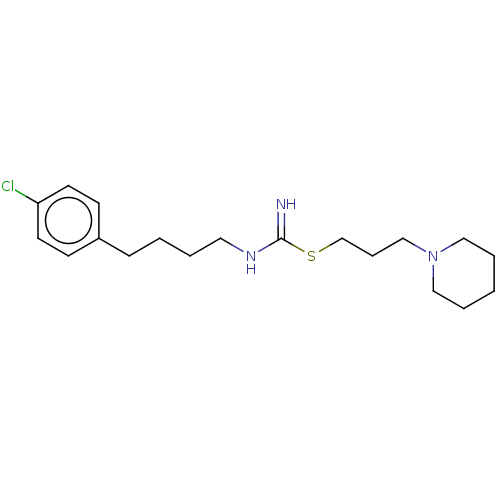
(CHEMBL2441942)Show InChI InChI=1S/C19H30ClN3S/c20-18-10-8-17(9-11-18)7-2-3-12-22-19(21)24-16-6-15-23-13-4-1-5-14-23/h8-11H,1-7,12-16H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494141
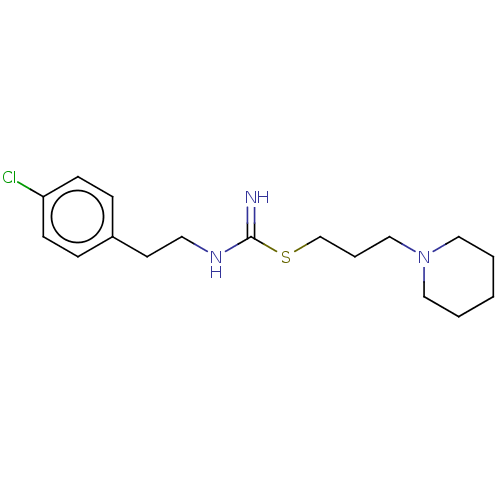
(CHEMBL2441940)Show InChI InChI=1S/C17H26ClN3S/c18-16-7-5-15(6-8-16)9-10-20-17(19)22-14-4-13-21-11-2-1-3-12-21/h5-8H,1-4,9-14H2,(H2,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494127

(CHEMBL2441944)Show SMILES Cl.FC(F)(F)c1ccc(CCCCNC(=N)SCCCN2CCCCC2)cc1 Show InChI InChI=1S/C20H30F3N3S/c21-20(22,23)18-10-8-17(9-11-18)7-2-3-12-25-19(24)27-16-6-15-26-13-4-1-5-14-26/h8-11H,1-7,12-16H2,(H2,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494135

(CHEMBL2441933)Show InChI InChI=1S/C15H22ClN3S/c16-14-6-4-13(5-7-14)12-18-15(17)20-11-3-10-19-8-1-2-9-19/h4-7H,1-3,8-12H2,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494131
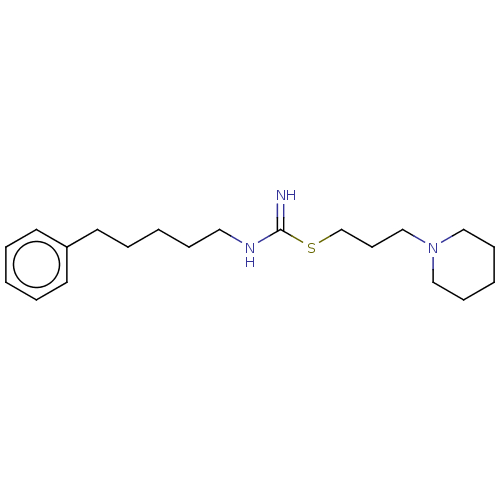
(CHEMBL2441943)Show InChI InChI=1S/C20H33N3S/c21-20(24-18-10-17-23-15-8-3-9-16-23)22-14-7-2-6-13-19-11-4-1-5-12-19/h1,4-5,11-12H,2-3,6-10,13-18H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494139

(CHEMBL2441938)Show InChI InChI=1S/C16H24ClN3S/c17-15-6-4-14(5-7-15)8-9-19-16(18)21-13-3-12-20-10-1-2-11-20/h4-7H,1-3,8-13H2,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494142
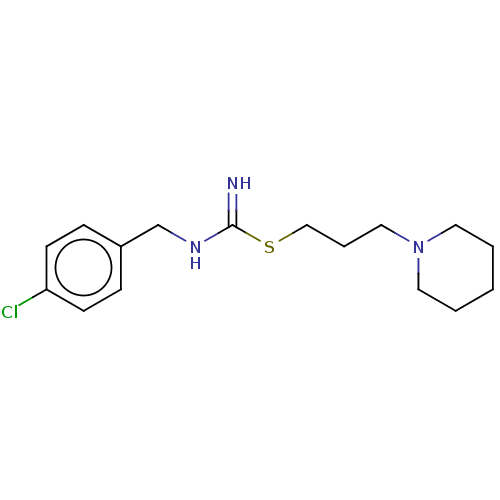
(CHEMBL2441935)Show InChI InChI=1S/C16H24ClN3S/c17-15-7-5-14(6-8-15)13-19-16(18)21-12-4-11-20-9-2-1-3-10-20/h5-8H,1-4,9-13H2,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM22372
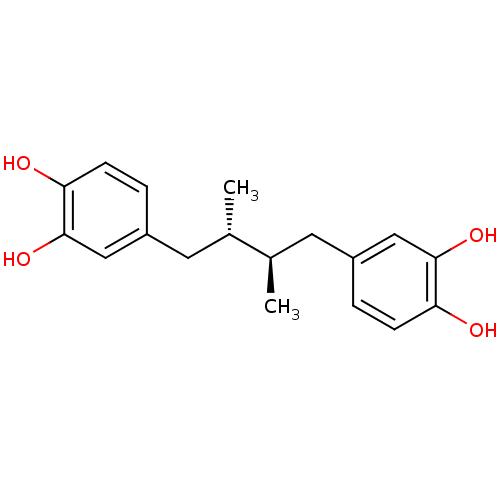
(4-[(2R,3S)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbut...)Show SMILES C[C@@H](Cc1ccc(O)c(O)c1)[C@H](C)Cc1ccc(O)c(O)c1 Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3/t11-,12+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494130

(CHEMBL2441947)Show InChI InChI=1S/C19H29ClN2O/c20-18-11-9-17(10-12-18)7-2-3-13-21-19(23)8-6-16-22-14-4-1-5-15-22/h9-12H,1-8,13-16H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494129

(CHEMBL2441948)Show InChI InChI=1S/C20H29F3N2O/c21-20(22,23)18-11-9-17(10-12-18)7-2-3-13-24-19(26)8-6-16-25-14-4-1-5-15-25/h9-12H,1-8,13-16H2,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM22372

(4-[(2R,3S)-4-(3,4-dihydroxyphenyl)-2,3-dimethylbut...)Show SMILES C[C@@H](Cc1ccc(O)c(O)c1)[C@H](C)Cc1ccc(O)c(O)c1 Show InChI InChI=1S/C18H22O4/c1-11(7-13-3-5-15(19)17(21)9-13)12(2)8-14-4-6-16(20)18(22)10-14/h3-6,9-12,19-22H,7-8H2,1-2H3/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50242010
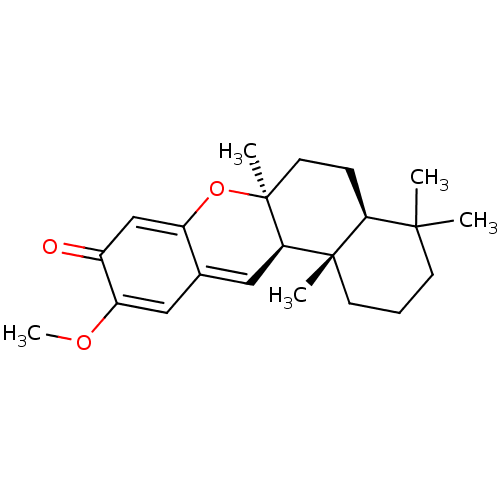
((+)-(5S,8S,9R,10S)-20-methoxypuupehenone | CHEMBL4...)Show SMILES COC1=CC2=C[C@H]3[C@](C)(CC[C@H]4C(C)(C)CCC[C@]34C)OC2=CC1=O |r,c:24,t:2,4| Show InChI InChI=1S/C22H30O3/c1-20(2)8-6-9-21(3)18(20)7-10-22(4)19(21)12-14-11-17(24-5)15(23)13-16(14)25-22/h11-13,18-19H,6-10H2,1-5H3/t18-,19+,21-,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50242017
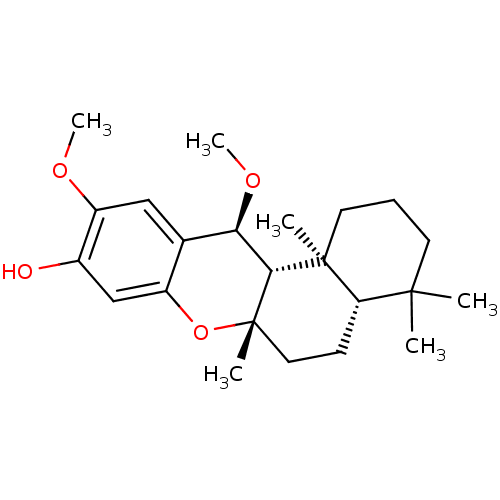
((+)-(5S,8S,10S)-20-methoxypuupehenol | (+)-(5S,8S,...)Show SMILES CO[C@H]1[C@H]2[C@](C)(CC[C@H]3C(C)(C)CCC[C@]23C)Oc2cc(O)c(OC)cc12 |r| Show InChI InChI=1S/C23H34O4/c1-21(2)9-7-10-22(3)18(21)8-11-23(4)20(22)19(26-6)14-12-17(25-5)15(24)13-16(14)27-23/h12-13,18-20,24H,7-11H2,1-6H3/t18-,19+,20+,22-,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50240936
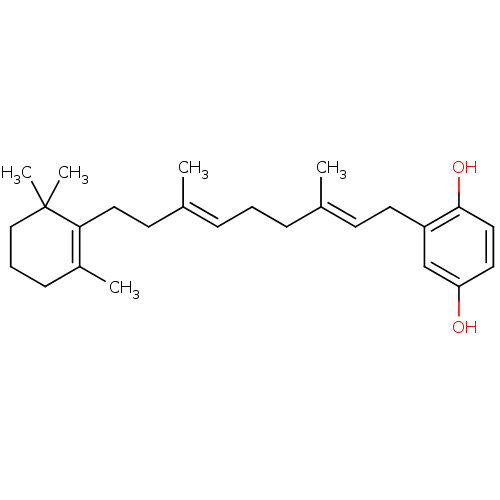
(2-[(2E,6E)-3,7-Dimethyl-9-(2,6,6-trimethyl-cyclohe...)Show SMILES C\C(CC\C=C(/C)CCC1=C(C)CCCC1(C)C)=C/Cc1cc(O)ccc1O |c:9| Show InChI InChI=1S/C26H38O2/c1-19(11-13-22-18-23(27)14-16-25(22)28)8-6-9-20(2)12-15-24-21(3)10-7-17-26(24,4)5/h9,11,14,16,18,27-28H,6-8,10,12-13,15,17H2,1-5H3/b19-11+,20-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494128
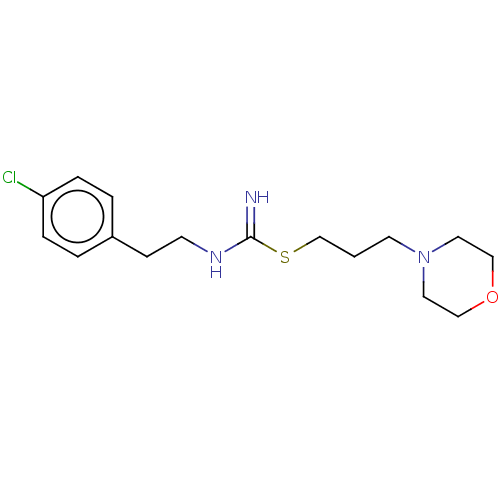
(CHEMBL2441939)Show InChI InChI=1S/C16H24ClN3OS/c17-15-4-2-14(3-5-15)6-7-19-16(18)22-13-1-8-20-9-11-21-12-10-20/h2-5H,1,6-13H2,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494126
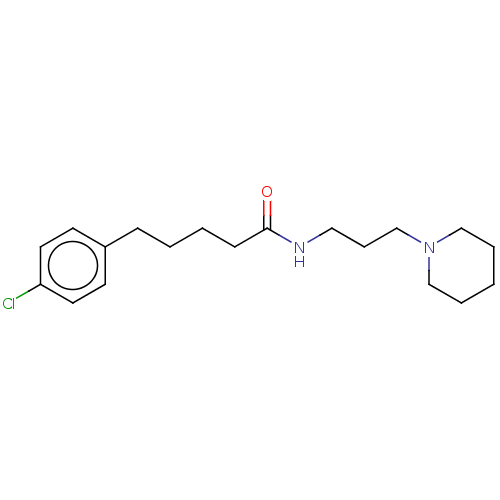
(CHEMBL2441949)Show InChI InChI=1S/C19H29ClN2O/c20-18-11-9-17(10-12-18)7-2-3-8-19(23)21-13-6-16-22-14-4-1-5-15-22/h9-12H,1-8,13-16H2,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50206259
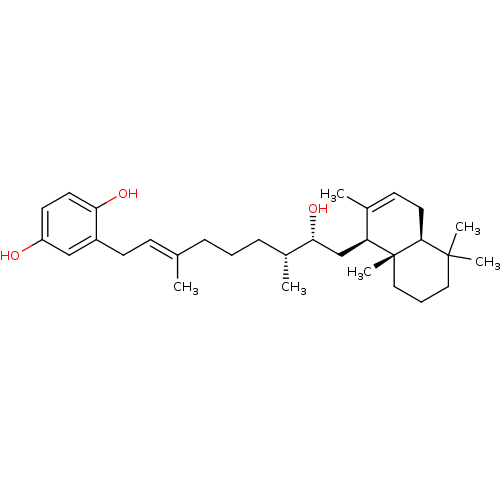
(CHEMBL230058 | hydrohalisulfate 1)Show SMILES C[C@H](CCC\C(C)=C\Cc1cc(O)ccc1O)[C@H](O)C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C |r,c:23| Show InChI InChI=1S/C31H48O3/c1-21(11-13-24-19-25(32)14-15-27(24)33)9-7-10-23(3)28(34)20-26-22(2)12-16-29-30(4,5)17-8-18-31(26,29)6/h11-12,14-15,19,23,26,28-29,32-34H,7-10,13,16-18,20H2,1-6H3/b21-11+/t23-,26+,28-,29+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50065997
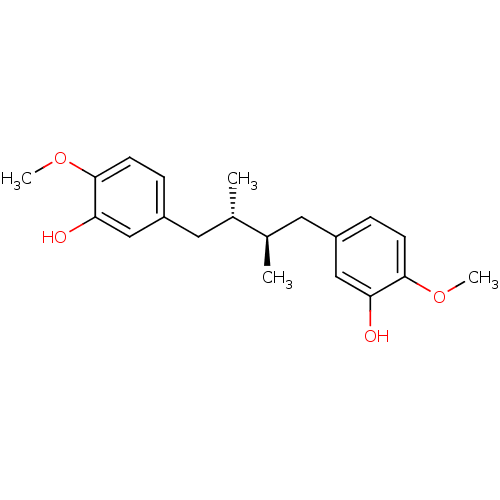
(5-[4-(3-hydroxy-4-methoxyphenyl)-2,3-dimethyl-(2R,...)Show SMILES COc1ccc(C[C@H](C)[C@H](C)Cc2ccc(OC)c(O)c2)cc1O |r| Show InChI InChI=1S/C20H26O4/c1-13(9-15-5-7-19(23-3)17(21)11-15)14(2)10-16-6-8-20(24-4)18(22)12-16/h5-8,11-14,21-22H,9-10H2,1-4H3/t13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578122

(CHEMBL4849848)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3cc(ccc3[nH]2)C(F)(F)F)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50240865

((4aS,6aS,12aR,12bS)-10-Hydroxy-4,4,6a,12b-tetramet...)Show SMILES CC1(C)CCC[C@@]2(C)[C@H]1CC[C@]1(C)OC3=CC(=O)C(=O)CC3=C[C@H]21 |r,c:23,t:15| Show InChI InChI=1S/C21H28O3/c1-19(2)7-5-8-20(3)17(19)6-9-21(4)18(20)11-13-10-14(22)15(23)12-16(13)24-21/h11-12,17-18H,5-10H2,1-4H3/t17-,18+,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50269763
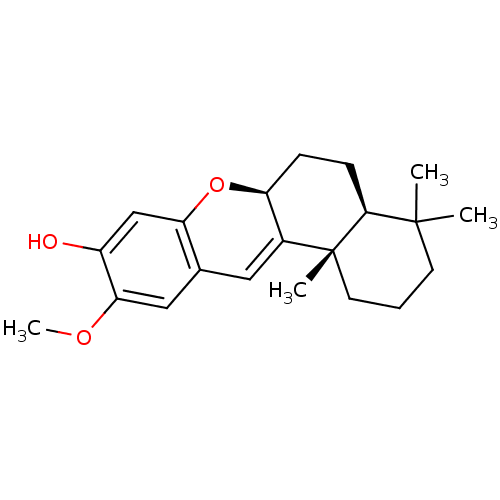
((+)-(5S,8S,10S)-20-methoxy-9,15-ene-puupehenol | C...)Show SMILES COc1cc2C=C3[C@H](CC[C@H]4C(C)(C)CCC[C@]34C)Oc2cc1O |r,c:5| Show InChI InChI=1S/C21H28O3/c1-20(2)8-5-9-21(3)14-10-13-11-18(23-4)15(22)12-17(13)24-16(14)6-7-19(20)21/h10-12,16,19,22H,5-9H2,1-4H3/t16-,19-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578109

(CHEMBL4863880)Show SMILES Cc1cc(C)cc(NC(=O)c2cncc(-c3cc(C)cc(C)c3)c2N2CCC(N)CC2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50269748
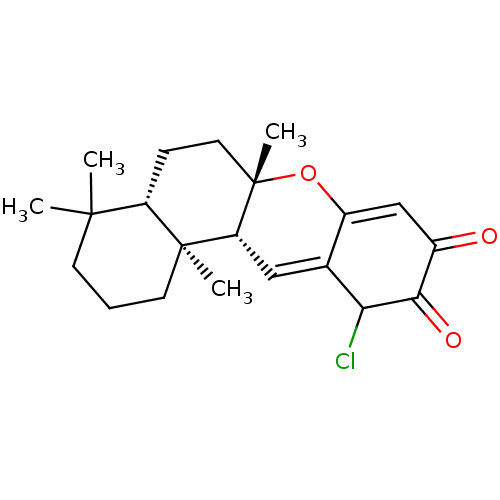
(21-chloro-puupehenone | CHEMBL465616 | chloropuupe...)Show SMILES CC1(C)CCC[C@@]2(C)[C@H]1CC[C@]1(C)OC3=CC(=O)C(=O)C(Cl)C3=C[C@H]21 |r,c:24,t:15| Show InChI InChI=1S/C21H27ClO3/c1-19(2)7-5-8-20(3)15(19)6-9-21(4)16(20)10-12-14(25-21)11-13(23)18(24)17(12)22/h10-11,15-17H,5-9H2,1-4H3/t15-,16+,17?,20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578113
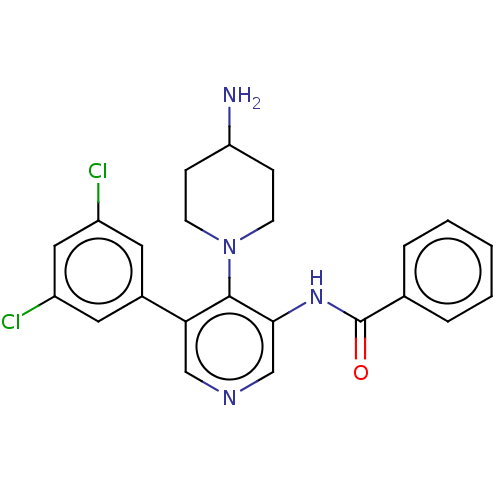
(CHEMBL4854239)Show SMILES NC1CCN(CC1)c1c(NC(=O)c2ccccc2)cncc1-c1cc(Cl)cc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50206257
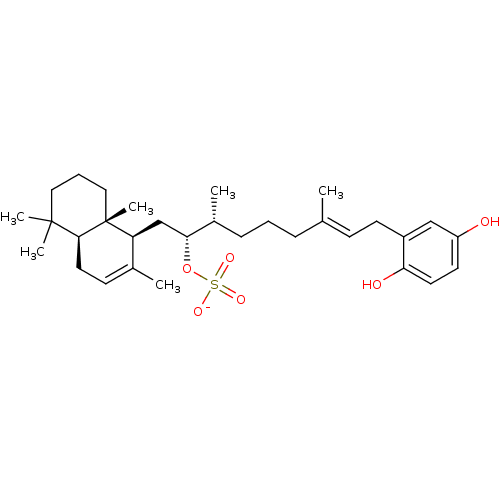
(CHEMBL387584 | CHEMBL450482 | halisulfate 1 | sodi...)Show SMILES C[C@H](CCC\C(C)=C\Cc1cc(O)ccc1O)[C@@H](C[C@H]1C(C)=CC[C@H]2C(C)(C)CCC[C@]12C)OS([O-])(=O)=O |r,c:22| Show InChI InChI=1S/C31H48O6S/c1-21(11-13-24-19-25(32)14-15-27(24)33)9-7-10-23(3)28(37-38(34,35)36)20-26-22(2)12-16-29-30(4,5)17-8-18-31(26,29)6/h11-12,14-15,19,23,26,28-29,32-33H,7-10,13,16-18,20H2,1-6H3,(H,34,35,36)/p-1/b21-11+/t23-,26+,28-,29+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578123
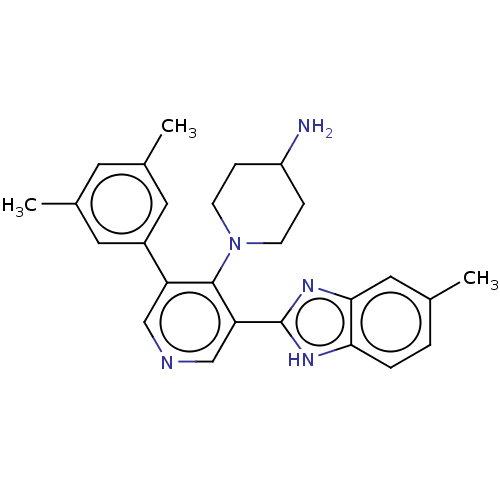
(CHEMBL4872591)Show SMILES Cc1ccc2[nH]c(nc2c1)-c1cncc(-c2cc(C)cc(C)c2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50269763

((+)-(5S,8S,10S)-20-methoxy-9,15-ene-puupehenol | C...)Show SMILES COc1cc2C=C3[C@H](CC[C@H]4C(C)(C)CCC[C@]34C)Oc2cc1O |r,c:5| Show InChI InChI=1S/C21H28O3/c1-20(2)8-5-9-21(3)14-10-13-11-18(23-4)15(22)12-17(13)24-16(14)6-7-19(20)21/h10-12,16,19,22H,5-9H2,1-4H3/t16-,19-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494134

(CHEMBL2441936)Show InChI InChI=1S/C16H25ClN4S/c1-20-8-10-21(11-9-20)7-2-12-22-16(18)19-13-14-3-5-15(17)6-4-14/h3-6H,2,7-13H2,1H3,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50240937
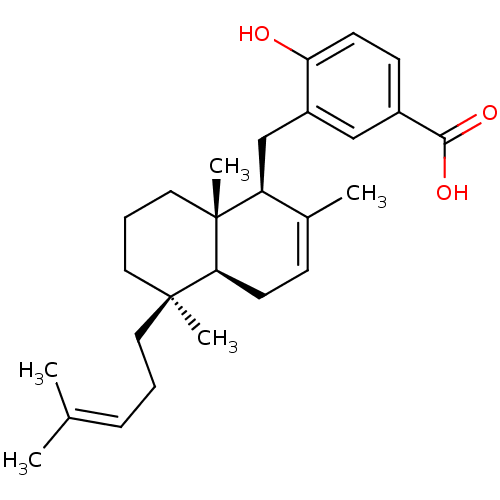
((-)-jaspic acid | 4-Hydroxy-3-[(1S,4aS,5R,8aS)-2,5...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-[#6][C@@]1([#6])[#6]-[#6]-[#6][C@]2([#6])[#6@@H](-[#6]-c3cc(ccc3-[#8])-[#6](-[#8])=O)-[#6](-[#6])=[#6]-[#6]-[#6@@H]12 |r,c:27| Show InChI InChI=1S/C27H38O3/c1-18(2)8-6-13-26(4)14-7-15-27(5)22(19(3)9-12-24(26)27)17-21-16-20(25(29)30)10-11-23(21)28/h8-11,16,22,24,28H,6-7,12-15,17H2,1-5H3,(H,29,30)/t22-,24-,26-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50494140

(CHEMBL2441934)Show InChI InChI=1S/C15H22ClN3OS/c16-14-4-2-13(3-5-14)12-18-15(17)21-11-1-6-19-7-9-20-10-8-19/h2-5H,1,6-12H2,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHO cells assessed as inhibition of forskolin/R-alpha-methylhistamine-induced cAMP ac... |
Bioorg Med Chem Lett 23: 6415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.052
BindingDB Entry DOI: 10.7270/Q2R78J5R |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50242010

((+)-(5S,8S,9R,10S)-20-methoxypuupehenone | CHEMBL4...)Show SMILES COC1=CC2=C[C@H]3[C@](C)(CC[C@H]4C(C)(C)CCC[C@]34C)OC2=CC1=O |r,c:24,t:2,4| Show InChI InChI=1S/C22H30O3/c1-20(2)8-6-9-21(3)18(20)7-10-22(4)19(21)12-14-11-17(24-5)15(23)13-16(14)25-22/h11-13,18-19H,6-10H2,1-5H3/t18-,19+,21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human reticulocyte 15-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578121
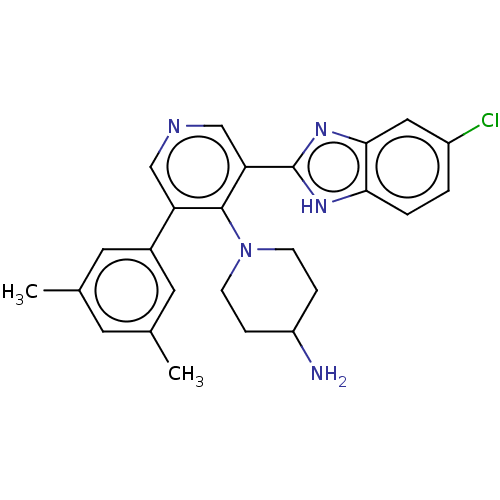
(CHEMBL4860633)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3cc(Cl)ccc3[nH]2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578110
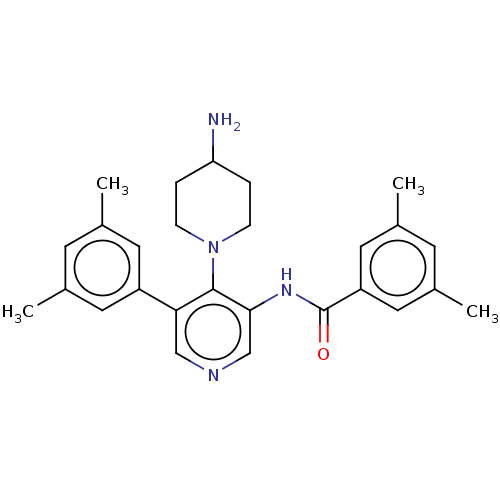
(CHEMBL4845867)Show SMILES Cc1cc(C)cc(c1)C(=O)Nc1cncc(-c2cc(C)cc(C)c2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578108
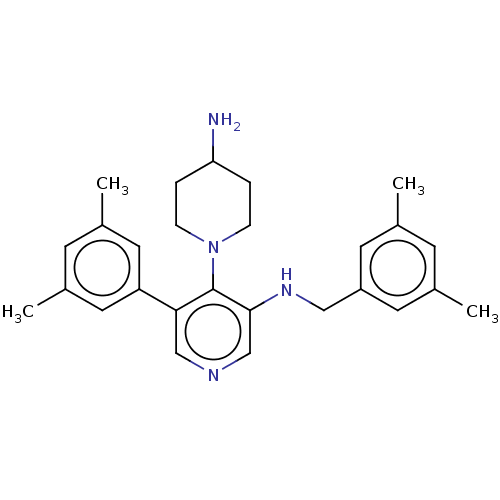
(CHEMBL4878248)Show SMILES Cc1cc(C)cc(CNc2cncc(-c3cc(C)cc(C)c3)c2N2CCC(N)CC2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578107
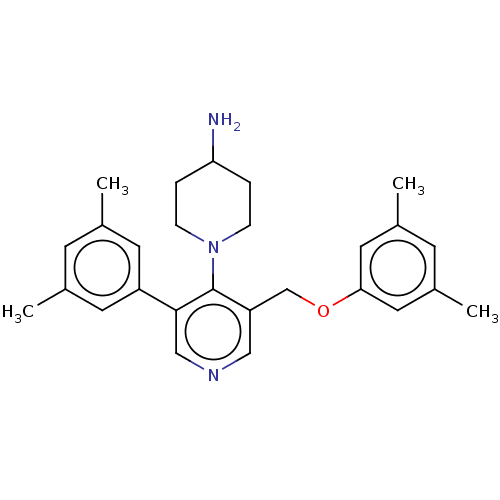
(CHEMBL4845931)Show SMILES Cc1cc(C)cc(OCc2cncc(-c3cc(C)cc(C)c3)c2N2CCC(N)CC2)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578114
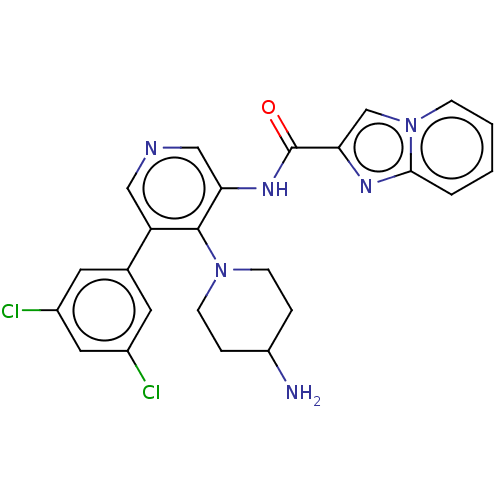
(CHEMBL4862717)Show SMILES NC1CCN(CC1)c1c(NC(=O)c2cn3ccccc3n2)cncc1-c1cc(Cl)cc(Cl)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578111

(CHEMBL4861425)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3cc(C)cc(C)c3[nH]2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578120
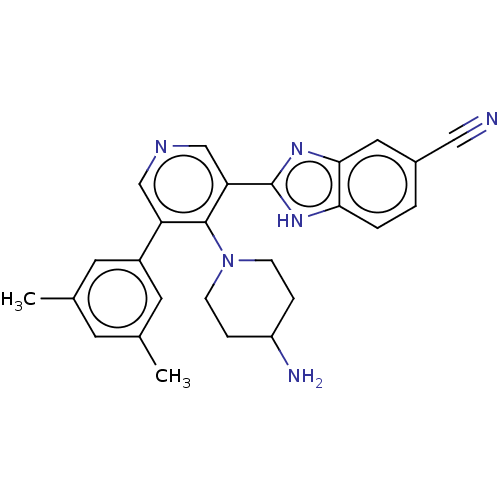
(CHEMBL4858892)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3cc(ccc3[nH]2)C#N)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50269748

(21-chloro-puupehenone | CHEMBL465616 | chloropuupe...)Show SMILES CC1(C)CCC[C@@]2(C)[C@H]1CC[C@]1(C)OC3=CC(=O)C(=O)C(Cl)C3=C[C@H]21 |r,c:24,t:15| Show InChI InChI=1S/C21H27ClO3/c1-19(2)7-5-8-20(3)15(19)6-9-21(4)16(20)10-12-14(25-21)11-13(23)18(24)17(12)22/h10-11,15-17H,5-9H2,1-4H3/t15-,16+,17?,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50240865

((4aS,6aS,12aR,12bS)-10-Hydroxy-4,4,6a,12b-tetramet...)Show SMILES CC1(C)CCC[C@@]2(C)[C@H]1CC[C@]1(C)OC3=CC(=O)C(=O)CC3=C[C@H]21 |r,c:23,t:15| Show InChI InChI=1S/C21H28O3/c1-19(2)7-5-8-20(3)17(19)6-9-21(4)18(20)11-13-10-14(22)15(23)12-16(13)24-21/h11-12,17-18H,5-10H2,1-4H3/t17-,18+,20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578119
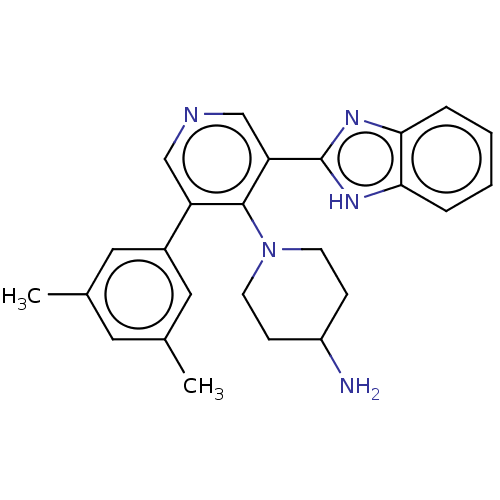
(CHEMBL4851978)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3ccccc3[nH]2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50065997

(5-[4-(3-hydroxy-4-methoxyphenyl)-2,3-dimethyl-(2R,...)Show SMILES COc1ccc(C[C@H](C)[C@H](C)Cc2ccc(OC)c(O)c2)cc1O |r| Show InChI InChI=1S/C20H26O4/c1-13(9-15-5-7-19(23-3)17(21)11-15)14(2)10-16-6-8-20(24-4)18(22)12-16/h5-8,11-14,21-22H,9-10H2,1-4H3/t13-,14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Santa Cruz
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged human platelet 12-lipoxygenase |
J Nat Prod 66: 230-5 (2003)
Article DOI: 10.1021/np020462l
BindingDB Entry DOI: 10.7270/Q25X29TQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578124
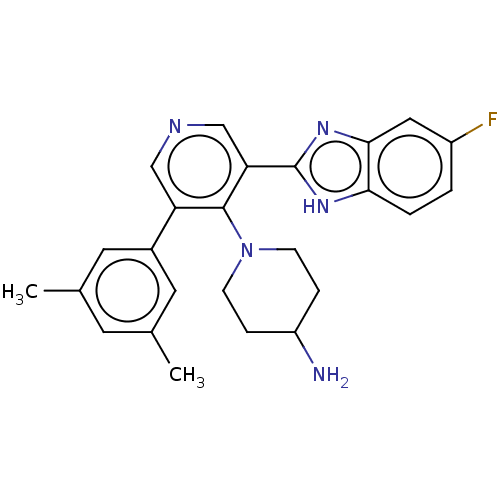
(CHEMBL4866325)Show SMILES Cc1cc(C)cc(c1)-c1cncc(-c2nc3cc(F)ccc3[nH]2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578125

(CHEMBL4878917)Show SMILES NC1CCN(CC1)c1c(cncc1-c1cc(F)cc(Cl)c1)-c1nc2cc(F)ccc2[nH]1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578118
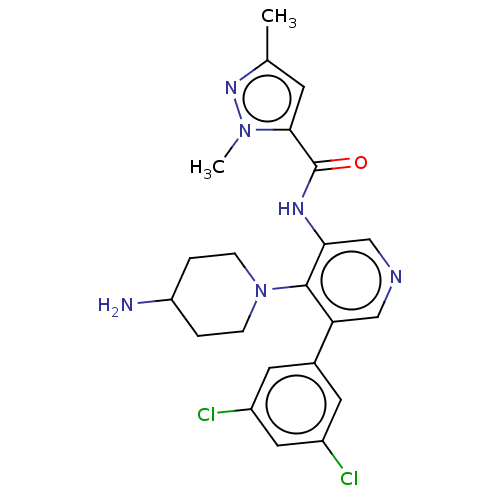
(CHEMBL4870834)Show SMILES Cc1cc(C(=O)Nc2cncc(-c3cc(Cl)cc(Cl)c3)c2N2CCC(N)CC2)n(C)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50578126
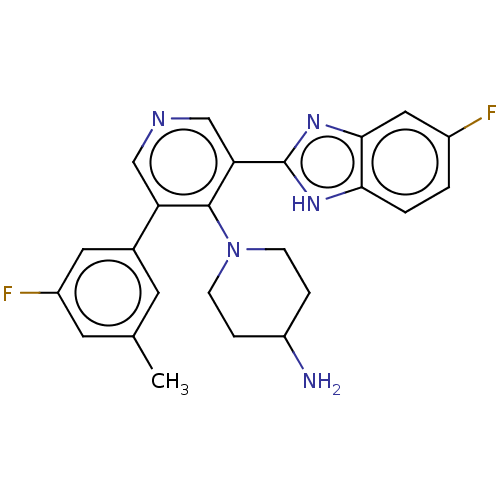
(CHEMBL4853282)Show SMILES Cc1cc(F)cc(c1)-c1cncc(-c2nc3cc(F)ccc3[nH]2)c1N1CCC(N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells with holding potential -80 mV incubated for 5 mins by patch-clamp assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116424
BindingDB Entry DOI: 10.7270/Q2CF9TXZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data