

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491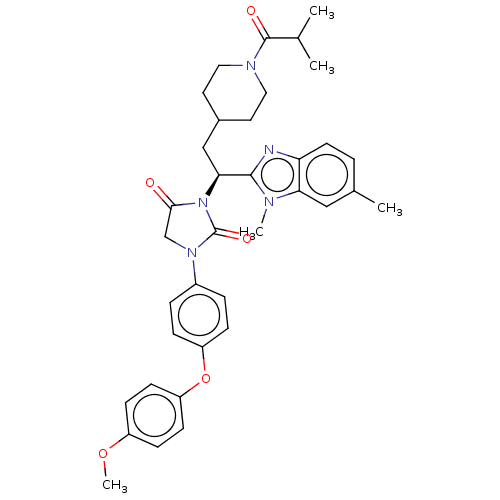 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286734 (CHEMBL4172988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510487 (CHEMBL4569266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286761 (CHEMBL4169187) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286736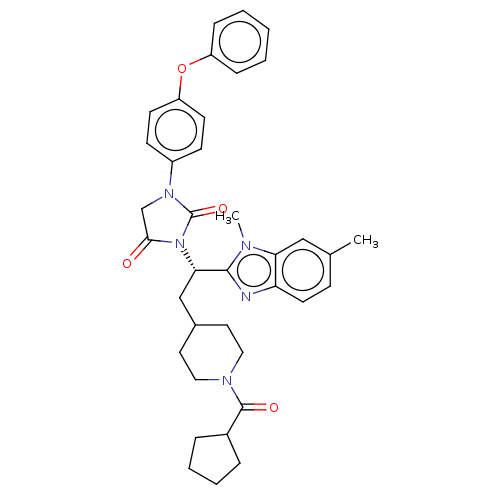 (CHEMBL4161262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286763 (CHEMBL4169596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286733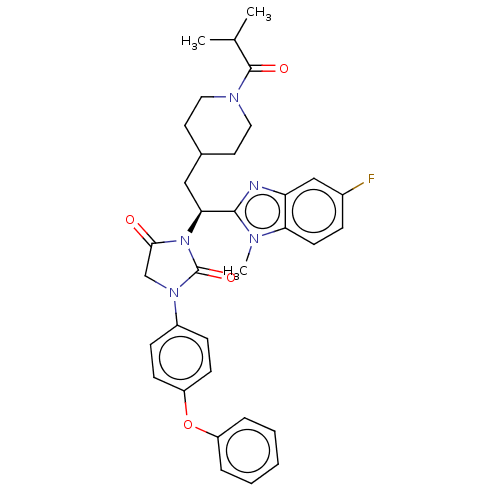 (CHEMBL4162312) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286762 (CHEMBL4159402) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286764 (CHEMBL4176369) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Hep3B cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity afte... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090510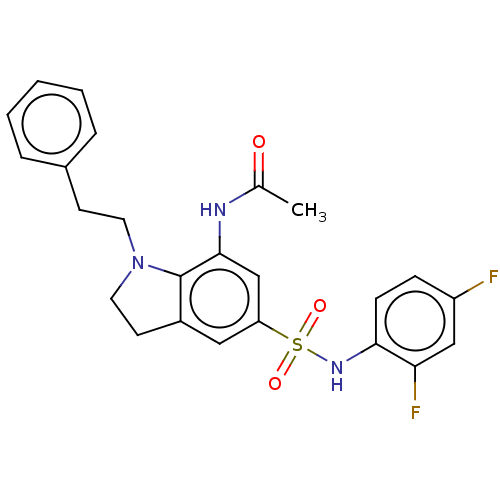 (CHEMBL3581716) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090666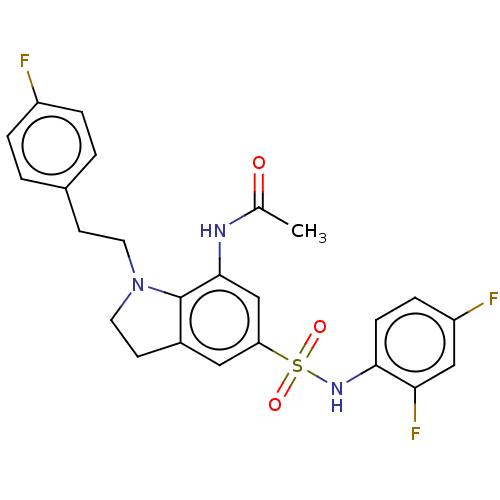 (CHEMBL3581717) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090665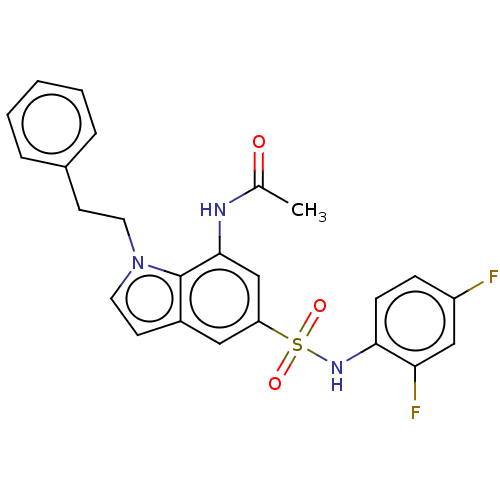 (CHEMBL3581718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090687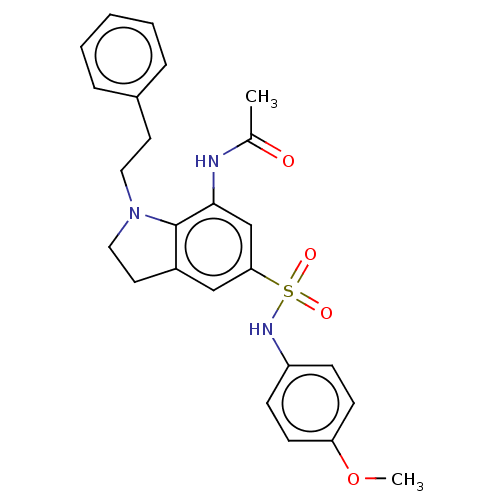 (CHEMBL3581715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090648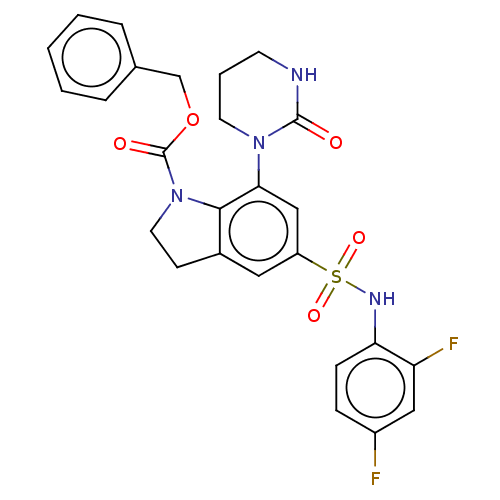 (CHEMBL3581732) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510488 (CHEMBL4519419) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090514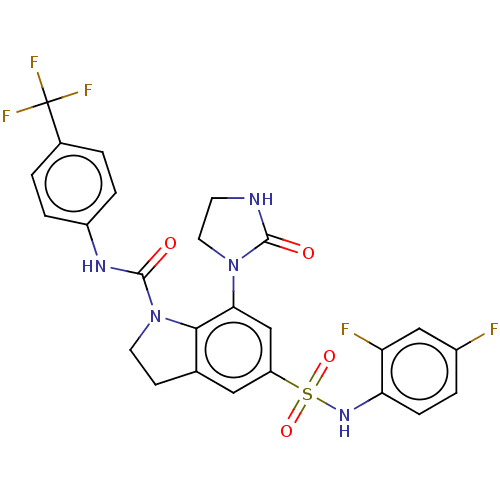 (CHEMBL3581736) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090617 (CHEMBL3581734) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090650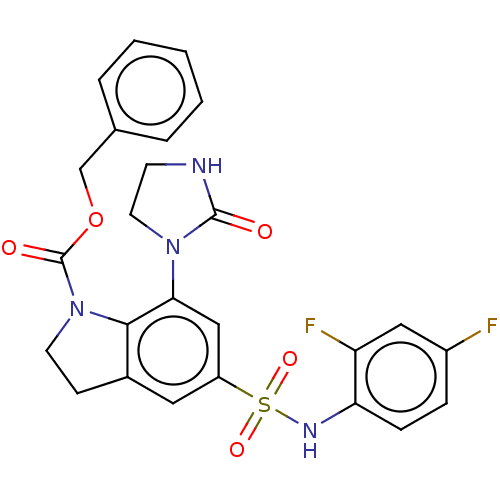 (CHEMBL3581730) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090616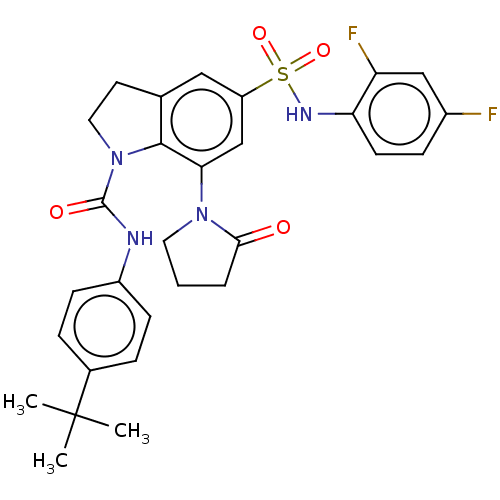 (CHEMBL3581735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090656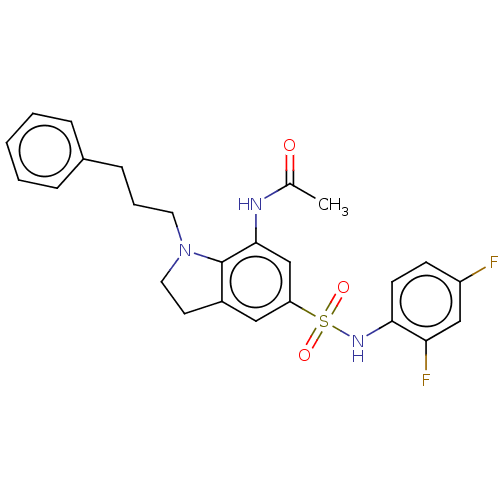 (CHEMBL3581724) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090653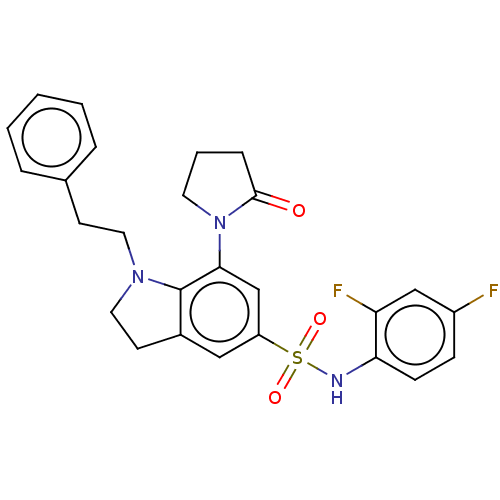 (CHEMBL3581727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510491 (CHEMBL4552760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090652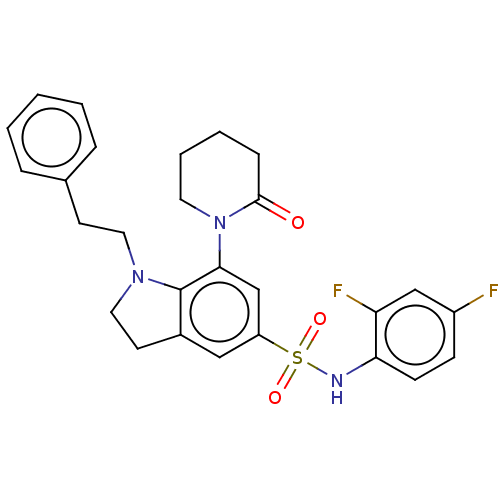 (CHEMBL3581728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510490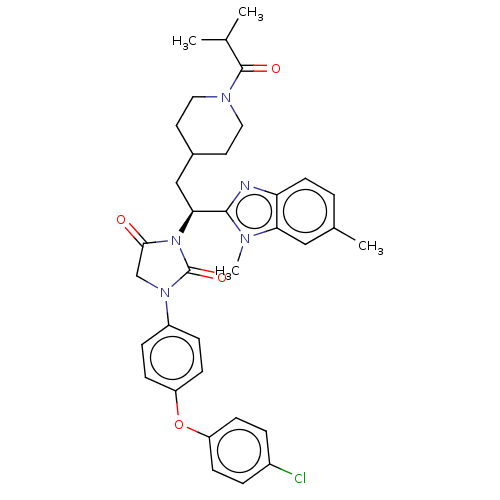 (CHEMBL4556659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090649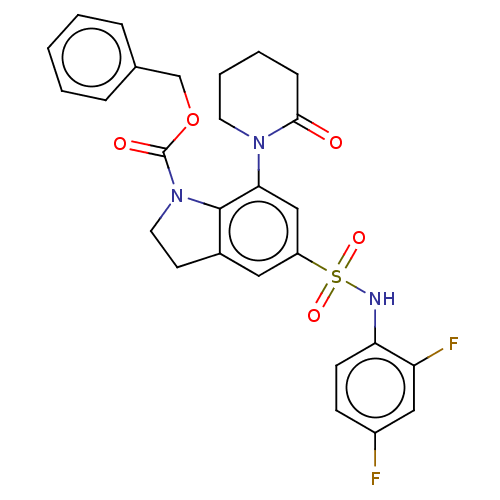 (CHEMBL3581731) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090651 (CHEMBL3581729) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510492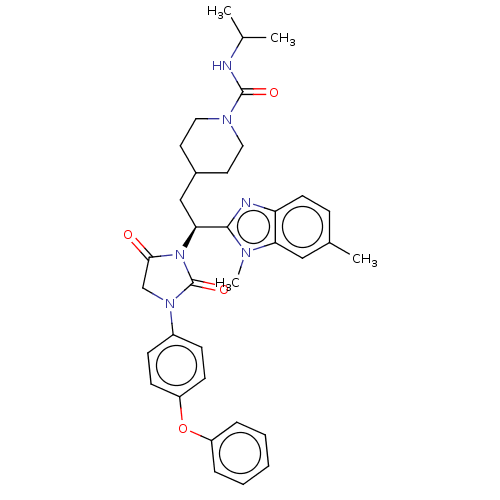 (CHEMBL4472554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090657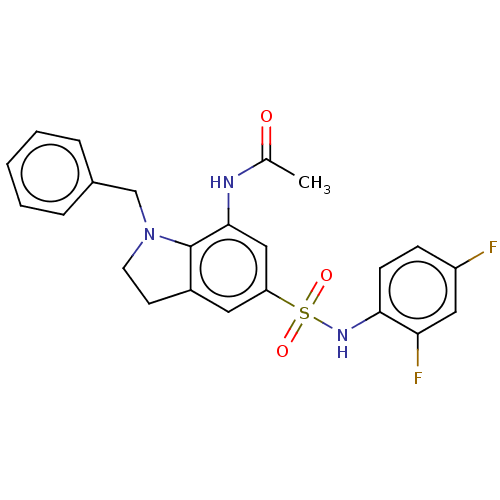 (CHEMBL3581723) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510490 (CHEMBL4556659) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090618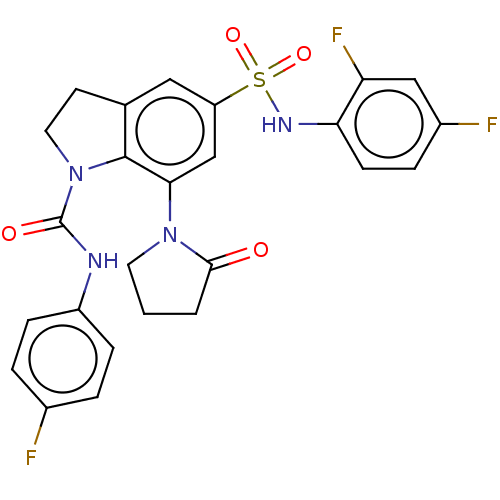 (CHEMBL3581733) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286736 (CHEMBL4161262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286760 (CHEMBL4170032) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510495 (CHEMBL4453417) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286734 (CHEMBL4172988) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510482 (CHEMBL4463196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090662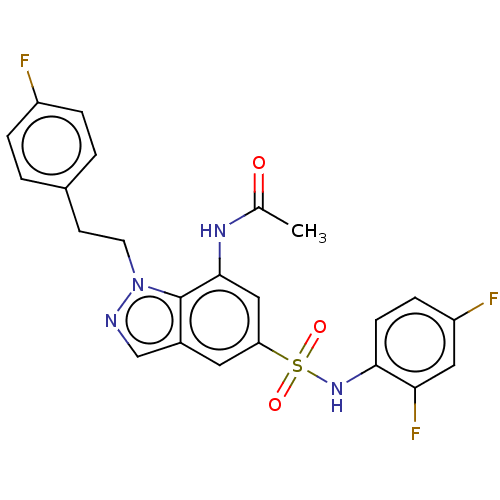 (CHEMBL3581719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090511 (CHEMBL3581739) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510487 (CHEMBL4569266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510479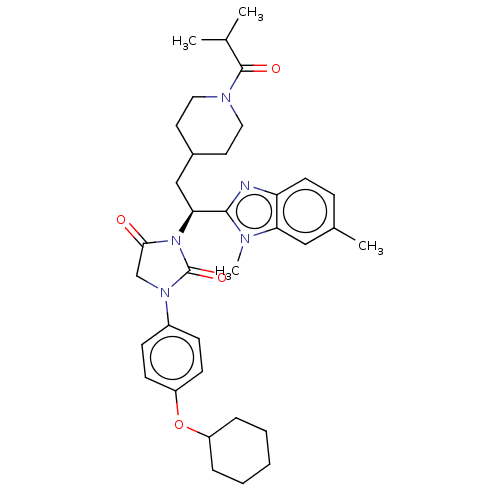 (CHEMBL4592488) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510486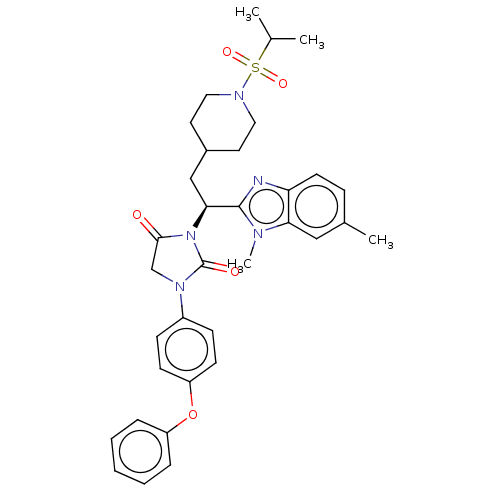 (CHEMBL4454185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50510497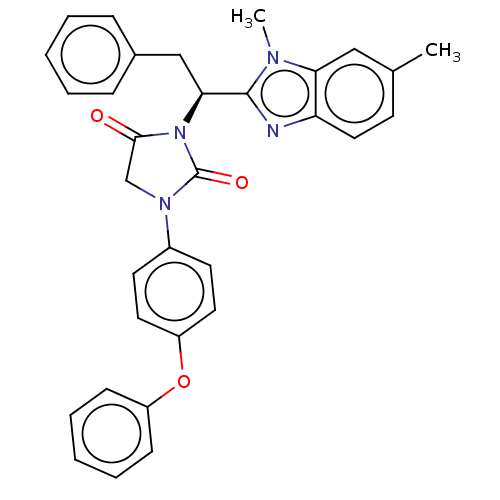 (CHEMBL4461640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at human FXR expressed in Huh7 cells assessed as inhibition of CDCA-induced FXR response element driven luciferase activity after... | Bioorg Med Chem 27: 2220-2227 (2019) Article DOI: 10.1016/j.bmc.2019.04.029 BindingDB Entry DOI: 10.7270/Q2R49V2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090512 (CHEMBL3581738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090513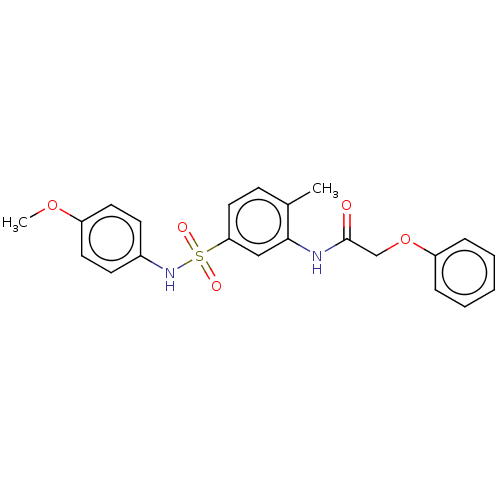 (CHEMBL3581737) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090654 (CHEMBL3581726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bile acid receptor (Homo sapiens (Human)) | BDBM50286763 (CHEMBL4169596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Hiroshima International University Curated by ChEMBL | Assay Description Antagonist activity at GST-tagged FXR LBD (unknown origin) assessed as inhibition of GW4064-induced fluorecein-labeled SRC2-2 coactivator recruitment... | ACS Med Chem Lett 9: 78-83 (2018) Article DOI: 10.1021/acsmedchemlett.7b00363 BindingDB Entry DOI: 10.7270/Q2GQ7199 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase (Homo sapiens (Human)) | BDBM50090661 (CHEMBL3581720) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibition of full length human MGAT2 transfected in FreeStyle293 cells assessed as dioleoylglycerol by RapidFire/MS assay | J Med Chem 58: 3892-909 (2015) Article DOI: 10.1021/acs.jmedchem.5b00178 BindingDB Entry DOI: 10.7270/Q2XK8H94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 199 total ) | Next | Last >> |