 Found 444 hits with Last Name = 'maniar' and Initial = 's'
Found 444 hits with Last Name = 'maniar' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510
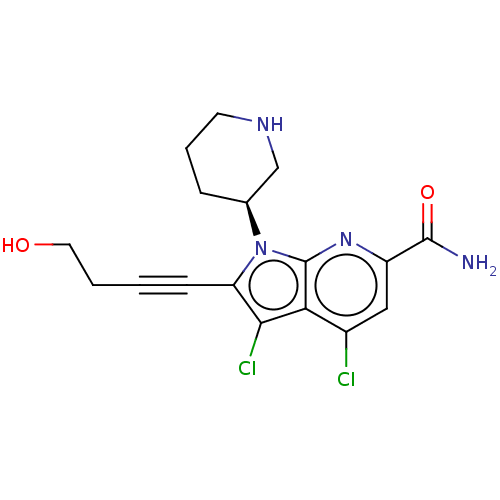
(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517
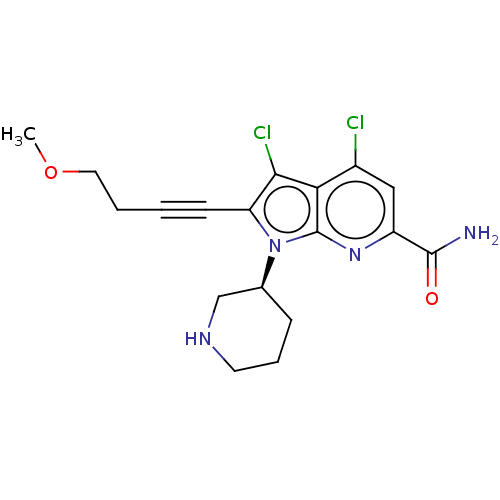
(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518507

(CHEMBL4583118)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CNCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H21Cl2N5O2/c20-13-8-14(18(22)27)25-19-16(13)17(21)15(4-3-12-10-24-6-7-28-12)26(19)11-2-1-5-23-9-11/h8,11-12,23-24H,1-2,5-7,9-10H2,(H2,22,27)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518514
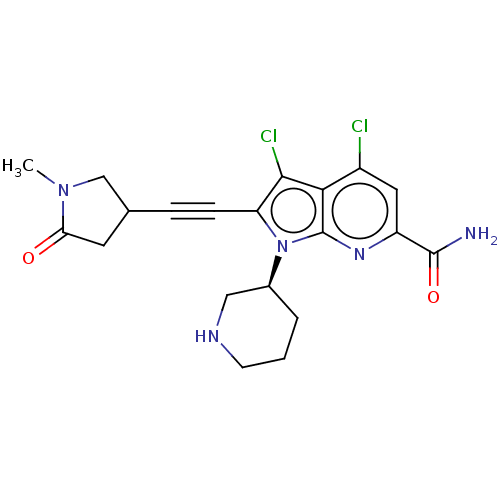
(CHEMBL4563802)Show SMILES CN1CC(CC1=O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H21Cl2N5O2/c1-26-10-11(7-16(26)28)4-5-15-18(22)17-13(21)8-14(19(23)29)25-20(17)27(15)12-3-2-6-24-9-12/h8,11-12,24H,2-3,6-7,9-10H2,1H3,(H2,23,29)/t11?,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518
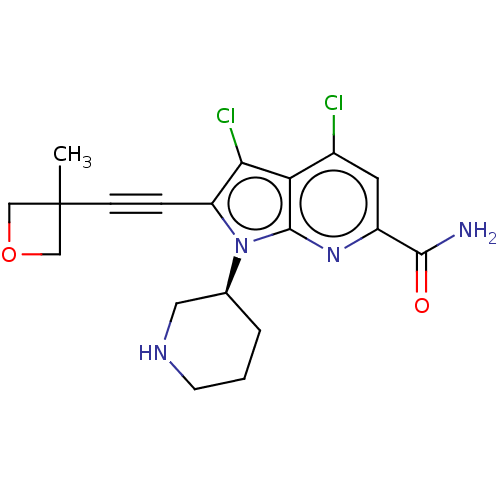
(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518505
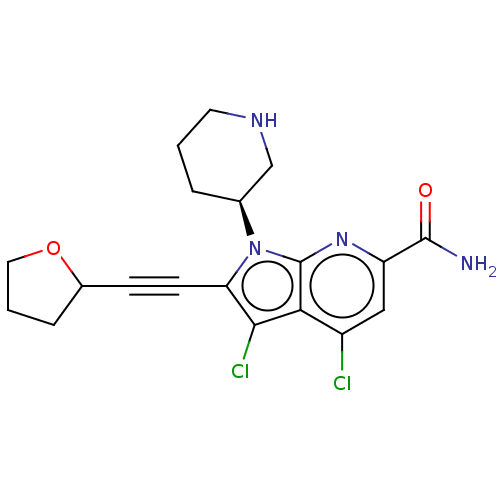
(CHEMBL4518492)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O2/c20-13-9-14(18(22)26)24-19-16(13)17(21)15(6-5-12-4-2-8-27-12)25(19)11-3-1-7-23-10-11/h9,11-12,23H,1-4,7-8,10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518522

(CHEMBL4466080)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C16H16Cl2N4O2/c17-10-7-11(15(19)24)21-16-13(10)14(18)12(4-2-6-23)22(16)9-3-1-5-20-8-9/h7,9,20,23H,1,3,5-6,8H2,(H2,19,24)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423687
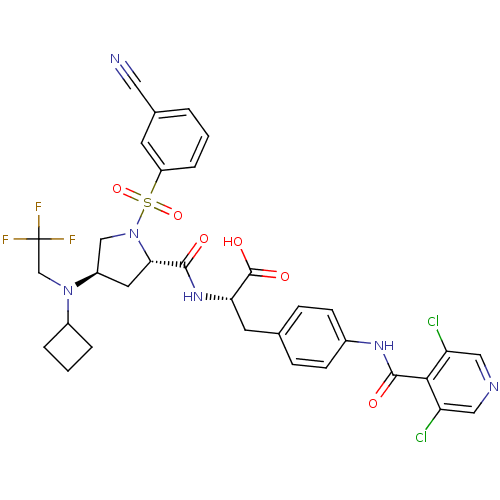
(CHEMBL569442)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N(CC(F)(F)F)C1CCC1 |r| Show InChI InChI=1S/C33H31Cl2F3N6O6S/c34-25-15-40-16-26(35)29(25)31(46)41-21-9-7-19(8-10-21)12-27(32(47)48)42-30(45)28-13-23(43(18-33(36,37)38)22-4-2-5-22)17-44(28)51(49,50)24-6-1-3-20(11-24)14-39/h1,3,6-11,15-16,22-23,27-28H,2,4-5,12-13,17-18H2,(H,41,46)(H,42,45)(H,47,48)/t23-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423695
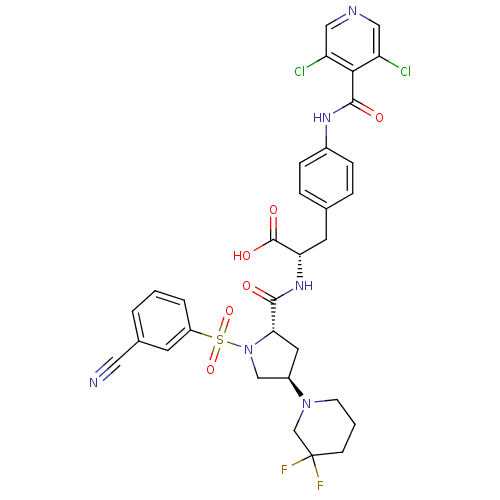
(CHEMBL566154 | MK-0617)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCC(F)(F)C1 |r| Show InChI InChI=1S/C32H30Cl2F2N6O6S/c33-24-15-38-16-25(34)28(24)30(44)39-21-7-5-19(6-8-21)12-26(31(45)46)40-29(43)27-13-22(41-10-2-9-32(35,36)18-41)17-42(27)49(47,48)23-4-1-3-20(11-23)14-37/h1,3-8,11,15-16,22,26-27H,2,9-10,12-13,17-18H2,(H,39,44)(H,40,43)(H,45,46)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing activating Mn2+ by competitive binding assay |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423695

(CHEMBL566154 | MK-0617)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCC(F)(F)C1 |r| Show InChI InChI=1S/C32H30Cl2F2N6O6S/c33-24-15-38-16-25(34)28(24)30(44)39-21-7-5-19(6-8-21)12-26(31(45)46)40-29(43)27-13-22(41-10-2-9-32(35,36)18-41)17-42(27)49(47,48)23-4-1-3-20(11-23)14-37/h1,3-8,11,15-16,22,26-27H,2,9-10,12-13,17-18H2,(H,39,44)(H,40,43)(H,45,46)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423695

(CHEMBL566154 | MK-0617)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCC(F)(F)C1 |r| Show InChI InChI=1S/C32H30Cl2F2N6O6S/c33-24-15-38-16-25(34)28(24)30(44)39-21-7-5-19(6-8-21)12-26(31(45)46)40-29(43)27-13-22(41-10-2-9-32(35,36)18-41)17-42(27)49(47,48)23-4-1-3-20(11-23)14-37/h1,3-8,11,15-16,22,26-27H,2,9-10,12-13,17-18H2,(H,39,44)(H,40,43)(H,45,46)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of VLA4 in human whole blood |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423692
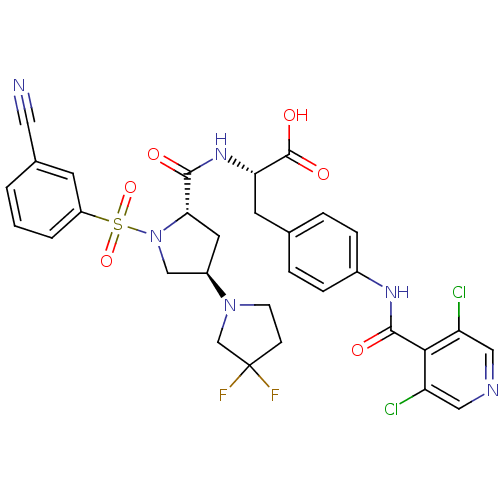
(CHEMBL569443)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCC(F)(F)C1 |r| Show InChI InChI=1S/C31H28Cl2F2N6O6S/c32-23-14-37-15-24(33)27(23)29(43)38-20-6-4-18(5-7-20)11-25(30(44)45)39-28(42)26-12-21(40-9-8-31(34,35)17-40)16-41(26)48(46,47)22-3-1-2-19(10-22)13-36/h1-7,10,14-15,21,25-26H,8-9,11-12,16-17H2,(H,38,43)(H,39,42)(H,44,45)/t21-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50309087
((S)-2-((2S,4R)-1-(3-cyanophenylsulfonyl)-4-(piperi...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCCC1 |r| Show InChI InChI=1S/C32H32Cl2N6O6S/c33-25-17-36-18-26(34)29(25)31(42)37-22-9-7-20(8-10-22)14-27(32(43)44)38-30(41)28-15-23(39-11-2-1-3-12-39)19-40(28)47(45,46)24-6-4-5-21(13-24)16-35/h4-10,13,17-18,23,27-28H,1-3,11-12,14-15,19H2,(H,37,42)(H,38,41)(H,43,44)/t23-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423690
(CHEMBL569276)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CC[C@@H](F)C1 |r| Show InChI InChI=1S/C31H29Cl2FN6O6S/c32-24-14-36-15-25(33)28(24)30(42)37-21-6-4-18(5-7-21)11-26(31(43)44)38-29(41)27-12-22(39-9-8-20(34)16-39)17-40(27)47(45,46)23-3-1-2-19(10-23)13-35/h1-7,10,14-15,20,22,26-27H,8-9,11-12,16-17H2,(H,37,42)(H,38,41)(H,43,44)/t20-,22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50309085

((S)-2-((2S,4R)-4-(azetidin-1-yl)-1-(3-cyanophenyls...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCC1 |r| Show InChI InChI=1S/C30H28Cl2N6O6S/c31-23-15-34-16-24(32)27(23)29(40)35-20-7-5-18(6-8-20)12-25(30(41)42)36-28(39)26-13-21(37-9-2-10-37)17-38(26)45(43,44)22-4-1-3-19(11-22)14-33/h1,3-8,11,15-16,21,25-26H,2,9-10,12-13,17H2,(H,35,40)(H,36,39)(H,41,42)/t21-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423686

(CHEMBL506044 | MK-0668)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)NC1CCC1 |r| Show InChI InChI=1S/C31H30Cl2N6O6S/c32-24-15-35-16-25(33)28(24)30(41)37-21-9-7-18(8-10-21)12-26(31(42)43)38-29(40)27-13-22(36-20-4-2-5-20)17-39(27)46(44,45)23-6-1-3-19(11-23)14-34/h1,3,6-11,15-16,20,22,26-27,36H,2,4-5,12-13,17H2,(H,37,41)(H,38,40)(H,42,43)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561654
(CHEMBL4785914)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)COC(F)(F)F)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561654

(CHEMBL4785914)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)COC(F)(F)F)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Mus musculus) | BDBM50561654

(CHEMBL4785914)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)COC(F)(F)F)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse UGT8 assessed as redcution in GalCer accumulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423693

(CHEMBL567433)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCC(F)C1 |r| Show InChI InChI=1S/C32H31Cl2FN6O6S/c33-25-15-37-16-26(34)29(25)31(43)38-22-8-6-19(7-9-22)12-27(32(44)45)39-30(42)28-13-23(40-10-2-4-21(35)17-40)18-41(28)48(46,47)24-5-1-3-20(11-24)14-36/h1,3,5-9,11,15-16,21,23,27-28H,2,4,10,12-13,17-18H2,(H,38,43)(H,39,42)(H,44,45)/t21?,23-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50309086
((S)-2-((3'R,5'S)-1'-(3-cyanophenylsulfonyl)-1,3'-b...)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCCC1 |r| Show InChI InChI=1S/C31H30Cl2N6O6S/c32-24-16-35-17-25(33)28(24)30(41)36-21-8-6-19(7-9-21)13-26(31(42)43)37-29(40)27-14-22(38-10-1-2-11-38)18-39(27)46(44,45)23-5-3-4-20(12-23)15-34/h3-9,12,16-17,22,26-27H,1-2,10-11,13-14,18H2,(H,36,41)(H,37,40)(H,42,43)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423696
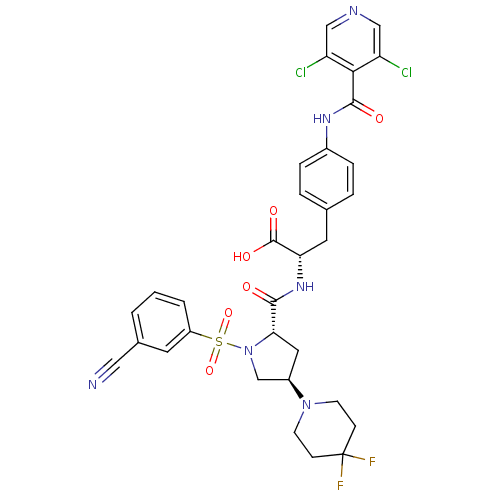
(CHEMBL569444)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCC(F)(F)CC1 |r| Show InChI InChI=1S/C32H30Cl2F2N6O6S/c33-24-16-38-17-25(34)28(24)30(44)39-21-6-4-19(5-7-21)13-26(31(45)46)40-29(43)27-14-22(41-10-8-32(35,36)9-11-41)18-42(27)49(47,48)23-3-1-2-20(12-23)15-37/h1-7,12,16-17,22,26-27H,8-11,13-14,18H2,(H,39,44)(H,40,43)(H,45,46)/t22-,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423691
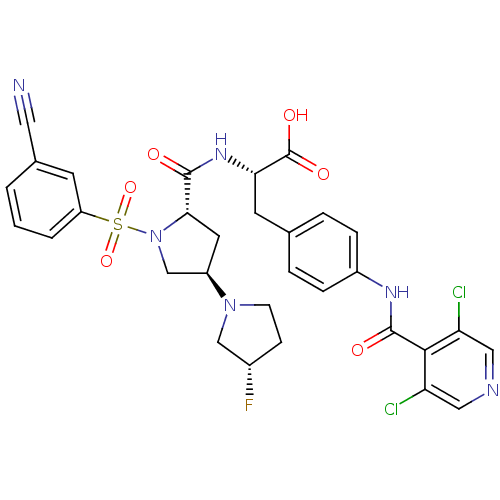
(CHEMBL569302)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CC[C@H](F)C1 |r| Show InChI InChI=1S/C31H29Cl2FN6O6S/c32-24-14-36-15-25(33)28(24)30(42)37-21-6-4-18(5-7-21)11-26(31(43)44)38-29(41)27-12-22(39-9-8-20(34)16-39)17-40(27)47(45,46)23-3-1-2-19(10-23)13-35/h1-7,10,14-15,20,22,26-27H,8-9,11-12,16-17H2,(H,37,42)(H,38,41)(H,43,44)/t20-,22+,26-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423688
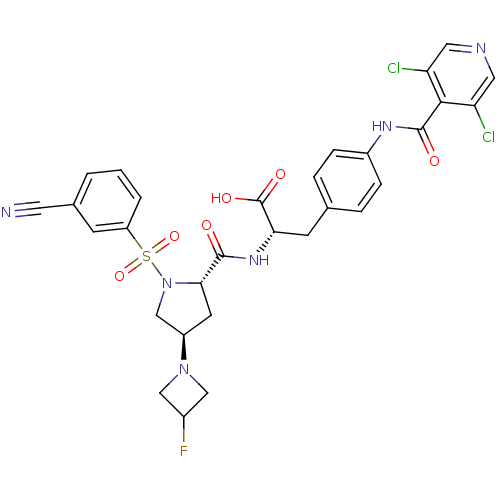
(CHEMBL571805)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CC(F)C1 |r| Show InChI InChI=1S/C30H27Cl2FN6O6S/c31-23-12-35-13-24(32)27(23)29(41)36-20-6-4-17(5-7-20)9-25(30(42)43)37-28(40)26-10-21(38-14-19(33)15-38)16-39(26)46(44,45)22-3-1-2-18(8-22)11-34/h1-8,12-13,19,21,25-26H,9-10,14-16H2,(H,36,41)(H,37,40)(H,42,43)/t21-,25+,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Mus musculus) | BDBM50561654

(CHEMBL4785914)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)COC(F)(F)F)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of mouse UGT8 assessed as redcution in SFT accumulation |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423694
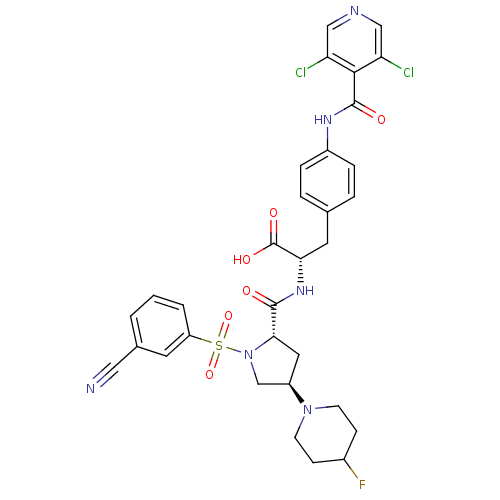
(CHEMBL565499)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CCC(F)CC1 |r| Show InChI InChI=1S/C32H31Cl2FN6O6S/c33-25-16-37-17-26(34)29(25)31(43)38-22-6-4-19(5-7-22)13-27(32(44)45)39-30(42)28-14-23(40-10-8-21(35)9-11-40)18-41(28)48(46,47)24-3-1-2-20(12-24)15-36/h1-7,12,16-17,21,23,27-28H,8-11,13-14,18H2,(H,38,43)(H,39,42)(H,44,45)/t23-,27+,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561669
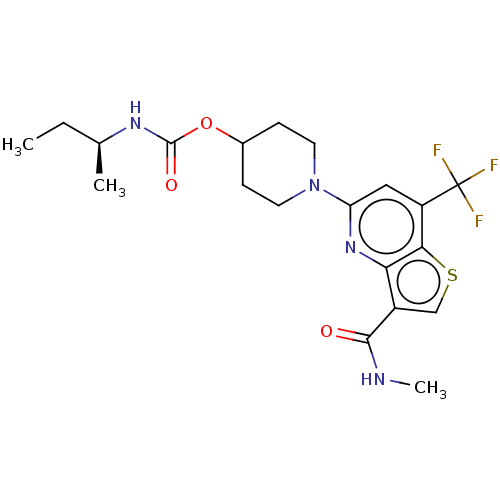
(CHEMBL4787939)Show SMILES CC[C@H](C)NC(=O)OC1CCN(CC1)c1cc(c2scc(C(=O)NC)c2n1)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561671

(CHEMBL4796521)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)NCCOC(F)(F)F)C(F)(F)F | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561671

(CHEMBL4796521)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)NCCOC(F)(F)F)C(F)(F)F | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518510

(CHEMBL4448325)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CCCO)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C17H18Cl2N4O2/c18-11-8-12(16(20)25)22-17-14(11)15(19)13(5-1-2-7-24)23(17)10-4-3-6-21-9-10/h8,10,21,24H,2-4,6-7,9H2,(H2,20,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518521

(CHEMBL4564586)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C18H18Cl2N4O/c19-12-8-13(17(21)25)23-18-15(12)16(20)14(6-5-10-3-4-10)24(18)11-2-1-7-22-9-11/h8,10-11,22H,1-4,7,9H2,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554405
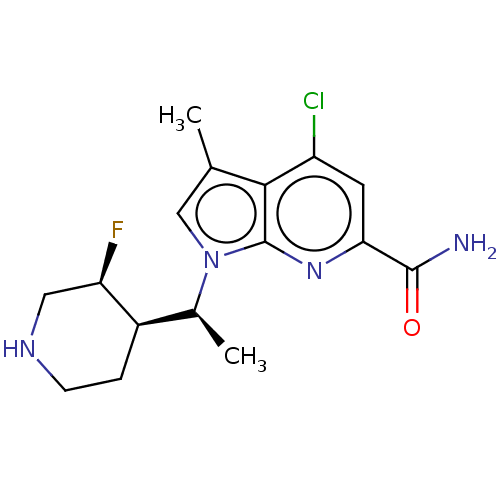
(CHEMBL4759685)Show SMILES [H][C@@]1(CCNC[C@H]1F)[C@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518517

(CHEMBL4540910)Show SMILES COCCC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-26-8-3-2-6-14-16(20)15-12(19)9-13(17(21)25)23-18(15)24(14)11-5-4-7-22-10-11/h9,11,22H,3-5,7-8,10H2,1H3,(H2,21,25)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561670
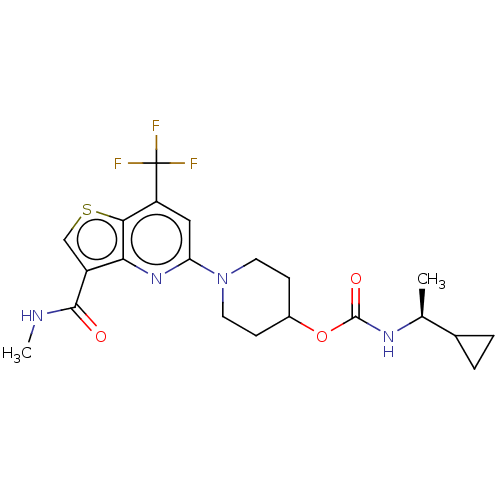
(CHEMBL4750073)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)C1CC1)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561669

(CHEMBL4787939)Show SMILES CC[C@H](C)NC(=O)OC1CCN(CC1)c1cc(c2scc(C(=O)NC)c2n1)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-4/beta-1
(Homo sapiens (Human)) | BDBM50423689
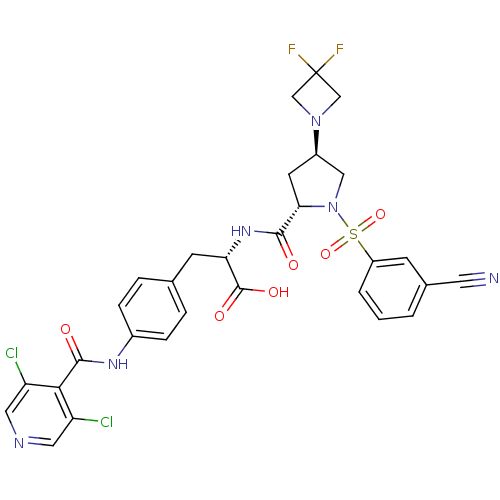
(CHEMBL569407)Show SMILES OC(=O)[C@H](Cc1ccc(NC(=O)c2c(Cl)cncc2Cl)cc1)NC(=O)[C@@H]1C[C@H](CN1S(=O)(=O)c1cccc(c1)C#N)N1CC(F)(F)C1 |r| Show InChI InChI=1S/C30H26Cl2F2N6O6S/c31-22-12-36-13-23(32)26(22)28(42)37-19-6-4-17(5-7-19)9-24(29(43)44)38-27(41)25-10-20(39-15-30(33,34)16-39)14-40(25)47(45,46)21-3-1-2-18(8-21)11-35/h1-8,12-13,20,24-25H,9-10,14-16H2,(H,37,42)(H,38,41)(H,43,44)/t20-,24+,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]-labeled ligand from VLA4 in human Jurkat cells washed with buffer containing non activating Ca2+ and Mg2+ by competitive bindin... |
Bioorg Med Chem Lett 19: 5803-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.111
BindingDB Entry DOI: 10.7270/Q2VQ33ZJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554401
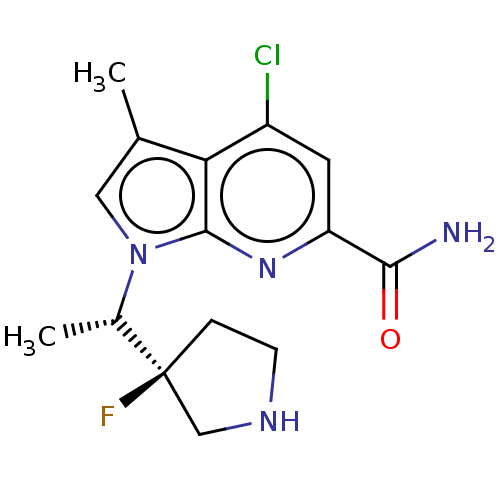
(CHEMBL4778578)Show SMILES C[C@H](n1cc(C)c2c(Cl)cc(nc12)C(N)=O)[C@]1(F)CCNC1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561664

(CHEMBL4776963)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)NCC1CC1)C(F)(F)F | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in SFT accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518506

(CHEMBL4588948)Show SMILES CC(O)CC#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C18H20Cl2N4O2/c1-10(25)4-2-6-14-16(20)15-12(19)8-13(17(21)26)23-18(15)24(14)11-5-3-7-22-9-11/h8,10-11,22,25H,3-5,7,9H2,1H3,(H2,21,26)/t10?,11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554403
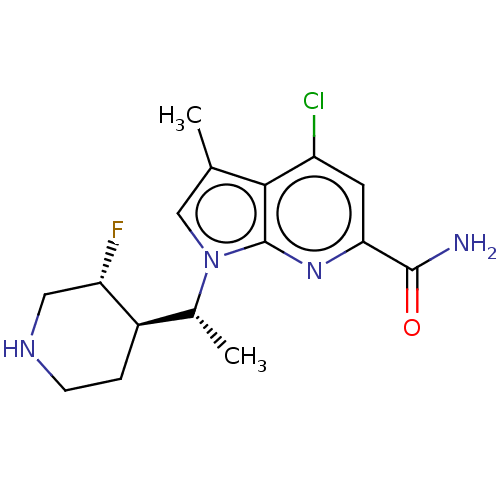
(CHEMBL4750046)Show SMILES [H][C@@]1(CCNC[C@@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50554404
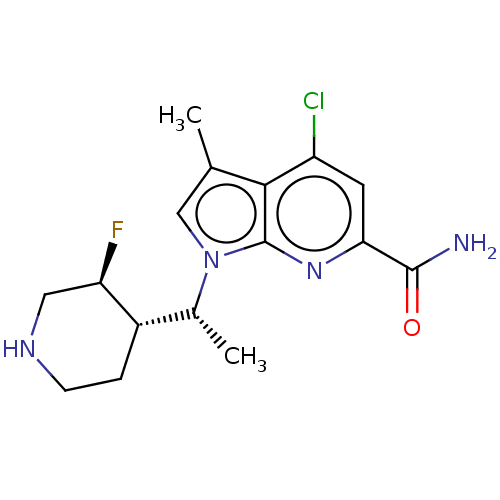
(CHEMBL4745141)Show SMILES [H][C@]1(CCNC[C@H]1F)[C@@H](C)n1cc(C)c2c(Cl)cc(nc12)C(N)=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PIM1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127625
BindingDB Entry DOI: 10.7270/Q2Z3239C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518520

(CHEMBL4593810)Show SMILES CC(O)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C17H18Cl2N4O2/c1-9(24)4-5-13-15(19)14-11(18)7-12(16(20)25)22-17(14)23(13)10-3-2-6-21-8-10/h7,9-10,21,24H,2-3,6,8H2,1H3,(H2,20,25)/t9?,10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
2-hydroxyacylsphingosine 1-beta-galactosyltransferase
(Homo sapiens) | BDBM50561670

(CHEMBL4750073)Show SMILES CNC(=O)c1csc2c(cc(nc12)N1CCC(CC1)OC(=O)N[C@@H](C)C1CC1)C(F)(F)F |r| | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of UGT8 in human OE19 cells assessed as redcution in GalCer accumulation measured after 72 hrs by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00120
BindingDB Entry DOI: 10.7270/Q2ZG6WX8 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518511
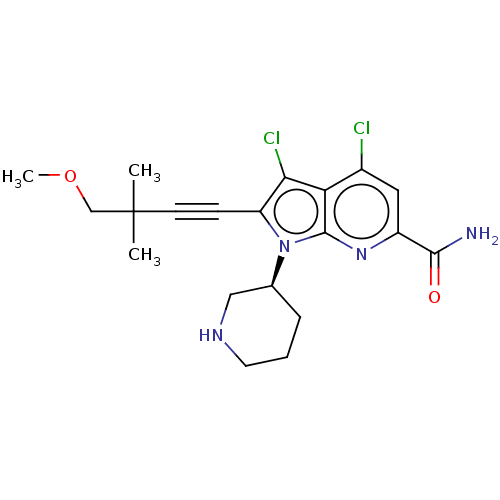
(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50518504
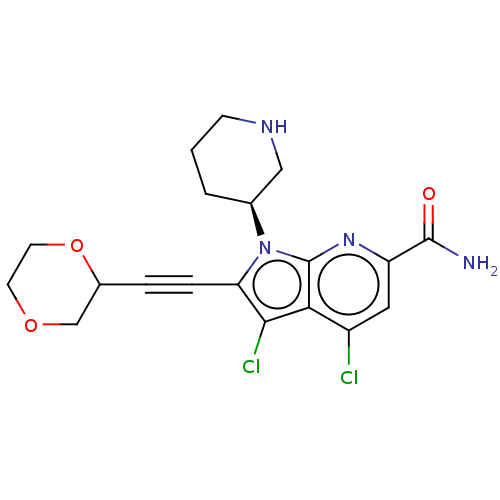
(CHEMBL4466764)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3COCCO3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C19H20Cl2N4O3/c20-13-8-14(18(22)26)24-19-16(13)17(21)15(4-3-12-10-27-6-7-28-12)25(19)11-2-1-5-23-9-11/h8,11-12,23H,1-2,5-7,9-10H2,(H2,22,26)/t11-,12?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518513

(CHEMBL4528544)Show SMILES NC(=O)c1cc(Cl)c2c(Cl)c(C#CC3CCOCC3)n([C@H]3CCCNC3)c2n1 |r| Show InChI InChI=1S/C20H22Cl2N4O2/c21-14-10-15(19(23)27)25-20-17(14)18(22)16(4-3-12-5-8-28-9-6-12)26(20)13-2-1-7-24-11-13/h10,12-13,24H,1-2,5-9,11H2,(H2,23,27)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518518

(CHEMBL4470576)Show SMILES CC1(COC1)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C19H20Cl2N4O2/c1-19(9-27-10-19)5-4-14-16(21)15-12(20)7-13(17(22)26)24-18(15)25(14)11-3-2-6-23-8-11/h7,11,23H,2-3,6,8-10H2,1H3,(H2,22,26)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50518511

(CHEMBL4457529)Show SMILES COCC(C)(C)C#Cc1c(Cl)c2c(Cl)cc(nc2n1[C@H]1CCCNC1)C(N)=O |r| Show InChI InChI=1S/C20H24Cl2N4O2/c1-20(2,11-28-3)7-6-15-17(22)16-13(21)9-14(18(23)27)25-19(16)26(15)12-5-4-8-24-10-12/h9,12,24H,4-5,8,10-11H2,1-3H3,(H2,23,27)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser-112 residue by TR-FRET assay |
Bioorg Med Chem Lett 29: 491-495 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.015
BindingDB Entry DOI: 10.7270/Q21V5J93 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data