

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM50460385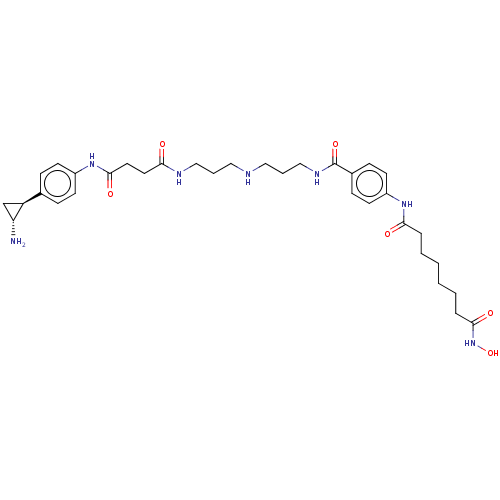 (CHEMBL4228572) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1/REST corepressor 3 (Homo sapiens (Human)) | BDBM50460386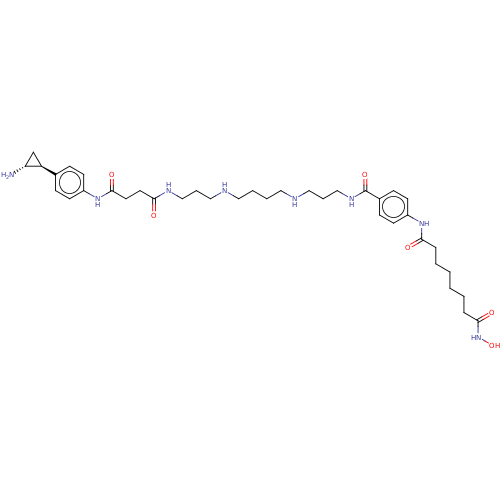 (CHEMBL4228166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC1/CoREST3 in HEK293 whole cell extract using fluorescent acetylated histone peptide as substrate after 60 mins by fluorescence base... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM11022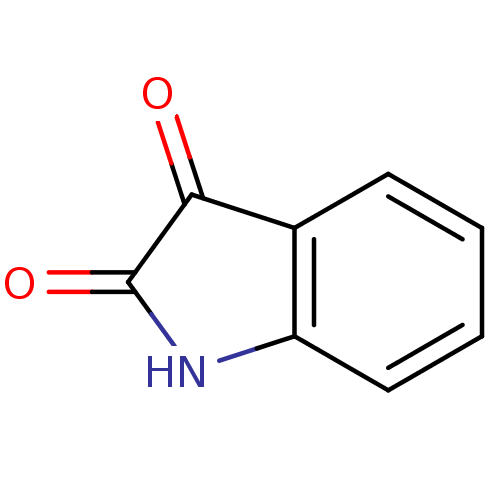 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of MAOA in human SH-SY5Y cells using p-tyramine as substrate preincubated for 5 mins by Lineweaver-Burk plot analysis | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Irreversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot ... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494371 (CHEMBL3086339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated f... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM11022 (2,3-dihydro-1H-indole-2,3-dione | CHEMBL326294 | I...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot a... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494370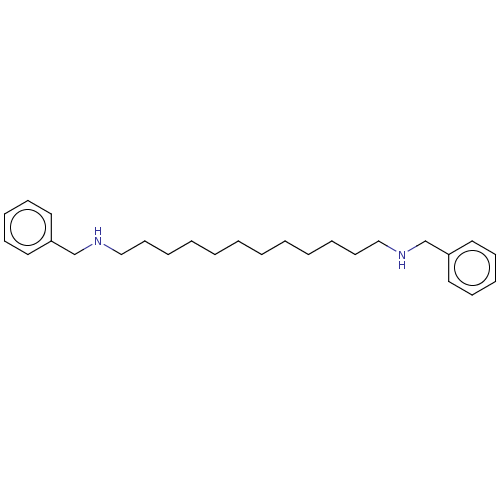 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.29E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.88E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.26E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.91E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494368 (CHEMBL1185605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50494369 (CHEMBL3086344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-A expressed in baculovirus infected BT1 cells using p-tyramine as substrate by Lineweaver-Burk plot an... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50494370 (CHEMBL3086341) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.05E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Padova Curated by ChEMBL | Assay Description Reversible inhibition of recombinant human MAO-B expressed in baculovirus infected BT1 cells using benzylamine as substrate at 200 uM preincubated fo... | Eur J Med Chem 70: 88-101 (2013) Article DOI: 10.1016/j.ejmech.2013.07.005 BindingDB Entry DOI: 10.7270/Q2T156MV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50062599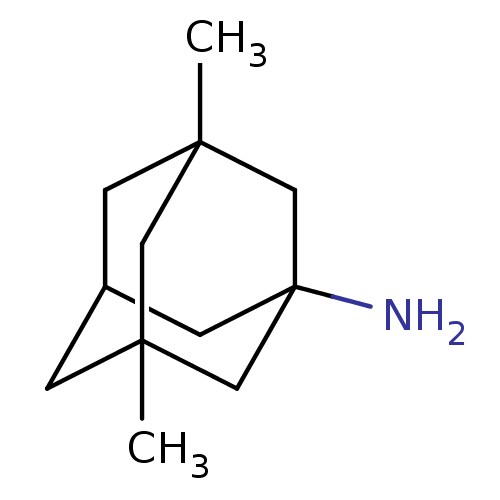 (3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50492253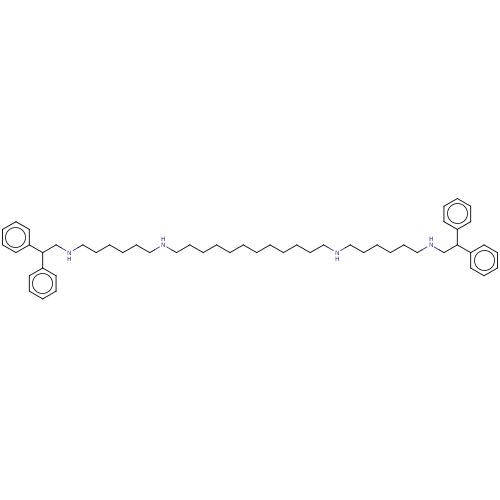 (CHEMBL2398522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50492252 (CHEMBL55568) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4567 (4-anilinoquinazoline deriv. 2 | BMC163482 Compound...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of EGFR-TK in human A431 cell lysate assessed as reduction in EGF stimulated kinase activity after 60 mins using biotinylated peptide subs... | Eur J Med Chem 117: 283-91 (2016) Article DOI: 10.1016/j.ejmech.2016.04.002 BindingDB Entry DOI: 10.7270/Q2V126QC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50285612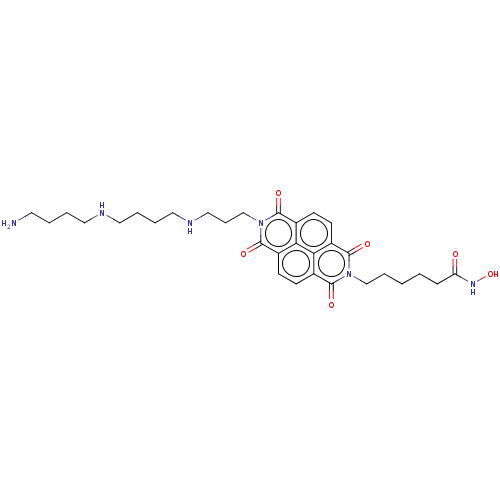 (CHEMBL4167599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged HDAC6 (1 to 1215 residues) expressed in baculovirus infected Sf21 insect cells using RHKKAc as ... | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50492255 (CHEMBL2009741) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50064176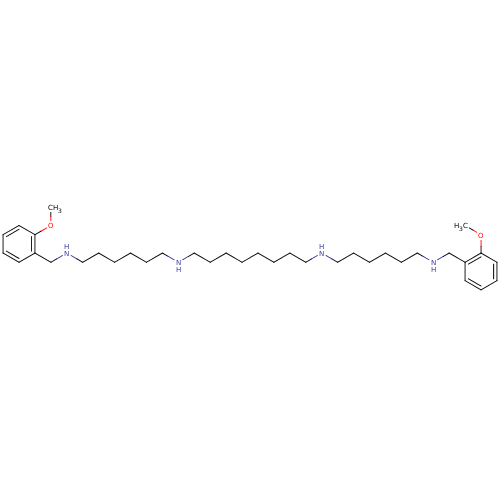 (CHEMBL27673 | CHEMBL500996 | METHOCTRAMINE | N,N''...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 138 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50492256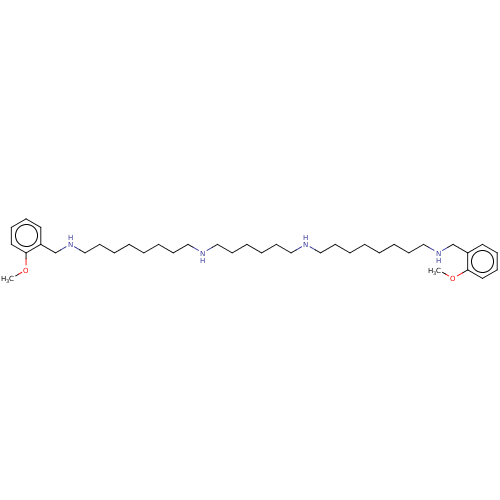 (CHEMBL2398521) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 1/2A (Homo sapiens (Human)) | BDBM50492254 (CHEMBL2398520) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 315 | n/a | n/a | n/a | n/a | n/a | n/a |
Chiba University Curated by ChEMBL | Assay Description Antagonist activity at recombinant GluN1/GluN2A receptor (unknown origin) expressed in Xenopus laevis oocytes assessed as inhibition of glycine/gluta... | Bioorg Med Chem Lett 23: 3901-4 (2013) Article DOI: 10.1016/j.bmcl.2013.04.063 BindingDB Entry DOI: 10.7270/Q2JM2DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50285611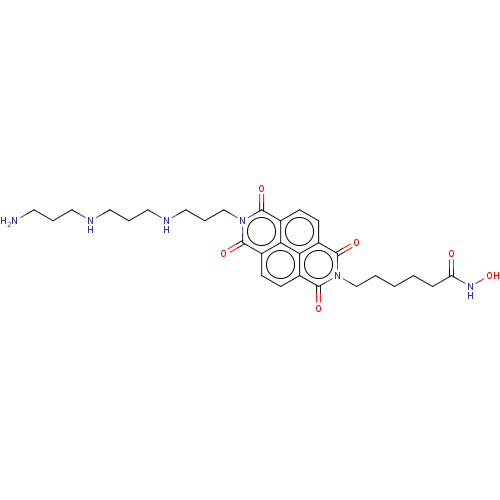 (CHEMBL4171407) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50285615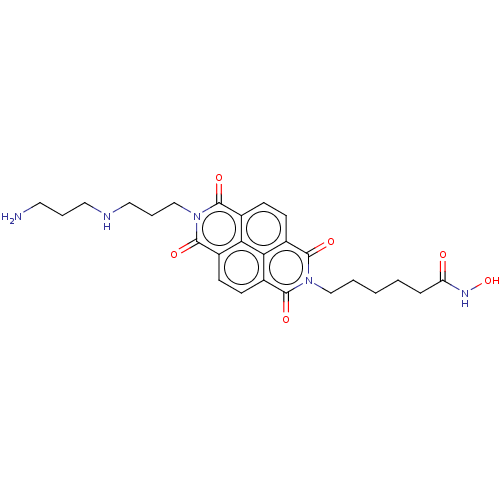 (CHEMBL4160757) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50285614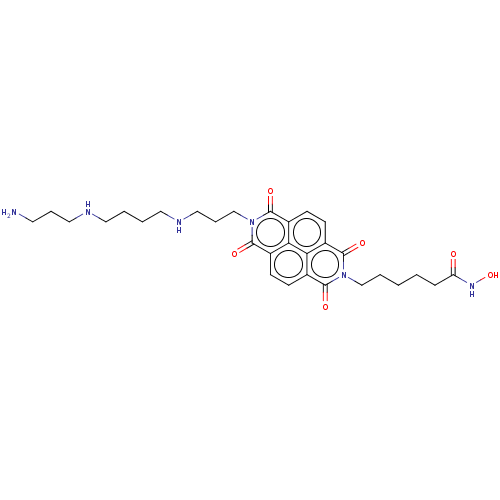 (CHEMBL4175475) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50285613 (CHEMBL4172024) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50285612 (CHEMBL4167599) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase (Homo sapiens (Human)) | BDBM50328678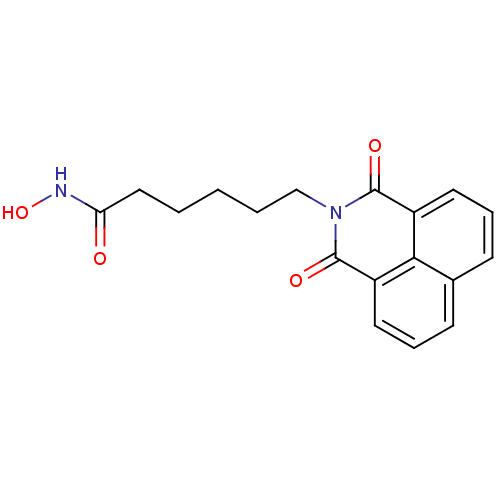 (6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of HDAC in human HeLa nuclear extract using Fluor de Lys as substrate by fluorimetric method | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50174346 (CHEMBL3809045) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of EGFR-TK in human A431 cell lysate assessed as reduction in EGF stimulated kinase activity after 60 mins using biotinylated peptide subs... | Eur J Med Chem 117: 283-91 (2016) Article DOI: 10.1016/j.ejmech.2016.04.002 BindingDB Entry DOI: 10.7270/Q2V126QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501616 (CHEMBL4084089) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501615 (CHEMBL4091846) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 3 (Homo sapiens) | BDBM50460385 (CHEMBL4228572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1/CoREST3 expressed in Escherichia coli using monomethylatedH3meK4 peptide as substrate preincubated for 15 mins f... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| REST corepressor 3 (Homo sapiens) | BDBM50460386 (CHEMBL4228166) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1/CoREST3 expressed in Escherichia coli using monomethylatedH3meK4 peptide as substrate preincubated for 15 mins f... | Bioorg Med Chem Lett 28: 1001-1004 (2018) Article DOI: 10.1016/j.bmcl.2018.02.034 BindingDB Entry DOI: 10.7270/Q2280B78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50285612 (CHEMBL4167599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal FLAG-His tagged HDAC1 (1 to 482 residues) expressed in Sf21 insect cells using RHK-K(Ac)-AMC as substrate ... | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501619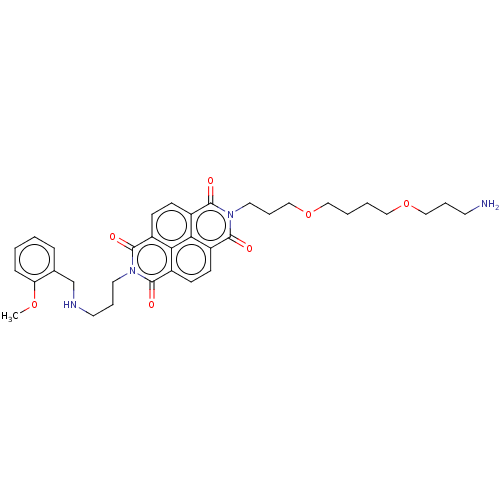 (CHEMBL4084827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501614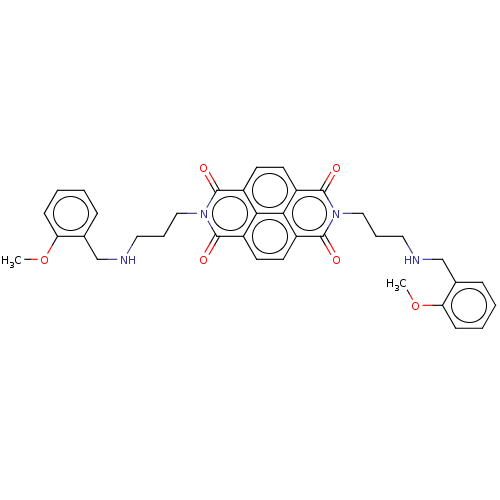 (CHEMBL583073) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3294 (4-Anilinoquinazoline deriv. 45 | 4-N-(3-bromopheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of EGFR-TK in human A431 cell lysate assessed as reduction in EGF stimulated kinase activity after 60 mins using biotinylated peptide subs... | Eur J Med Chem 117: 283-91 (2016) Article DOI: 10.1016/j.ejmech.2016.04.002 BindingDB Entry DOI: 10.7270/Q2V126QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501618 (CHEMBL4064888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50501617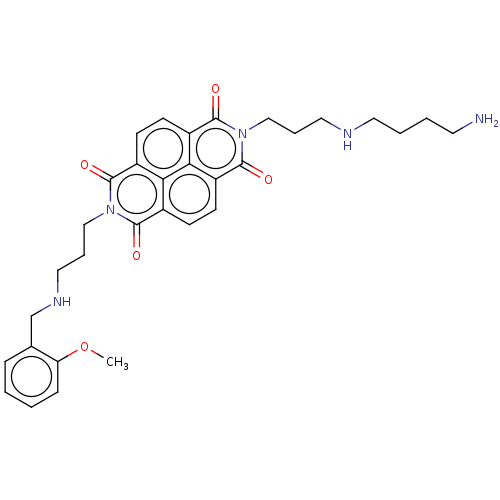 (CHEMBL4092588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of human topoisomerase-2alpha-mediated relaxation of supercoiled pBR322 DNA after 1 hr by ethidium bromide staining based agarose gel elec... | Eur J Med Chem 128: 107-122 (2017) Article DOI: 10.1016/j.ejmech.2017.01.025 BindingDB Entry DOI: 10.7270/Q2668H7X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50073654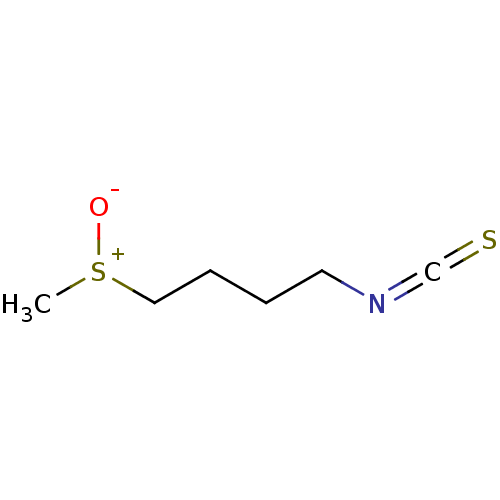 (4-(Methylsulfinyl)Butyl Isothiocyanate | CHEBI:478...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of EGFR-TK in human A431 cell lysate assessed as reduction in EGF stimulated kinase activity after 60 mins using biotinylated peptide subs... | Eur J Med Chem 117: 283-91 (2016) Article DOI: 10.1016/j.ejmech.2016.04.002 BindingDB Entry DOI: 10.7270/Q2V126QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 2 (Homo sapiens (Human)) | BDBM50285612 (CHEMBL4167599) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal GST-tagged HDAC2 (1 to 488 residues) expressed in baculovirus infected Sf21 insect cells using RHK-K(Ac)-A... | ACS Med Chem Lett 8: 1218-1223 (2017) Article DOI: 10.1021/acsmedchemlett.7b00289 BindingDB Entry DOI: 10.7270/Q22N54T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |