 Found 46 hits with Last Name = 'martín-santamaría' and Initial = 's'
Found 46 hits with Last Name = 'martín-santamaría' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10592
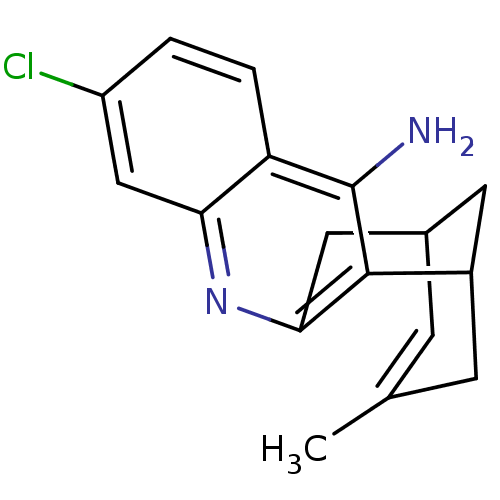
(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10597
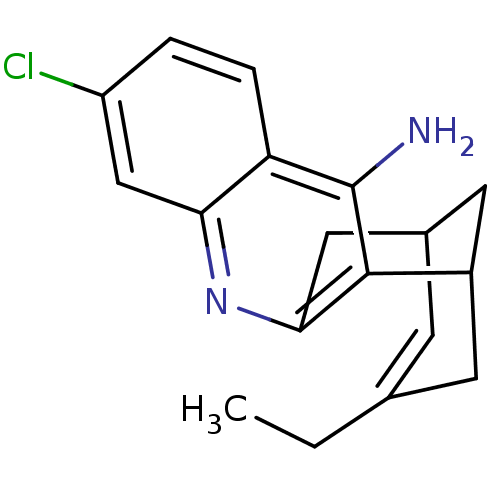
((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM10592

(7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...)Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM10597

((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:2,TLB:11:9:5:3.2.7,19:8:5:3.2.7| Show InChI InChI=1S/C18H19ClN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987
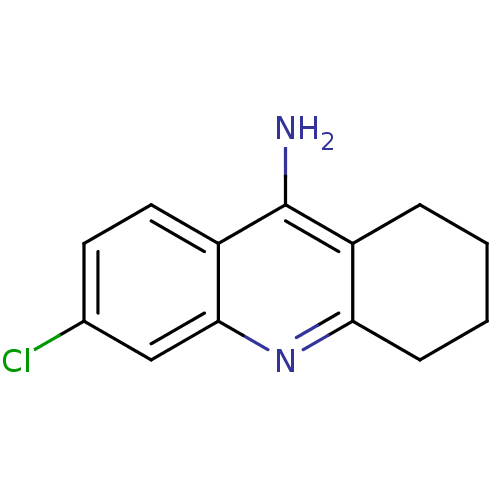
(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50094626
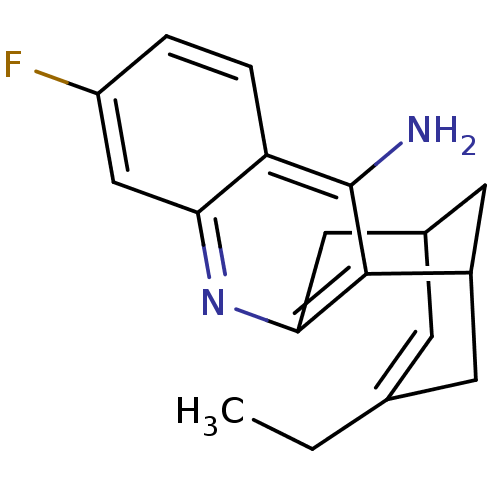
((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:2,TLB:19:8:2.3.7:5| Show InChI InChI=1S/C18H19FN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079861
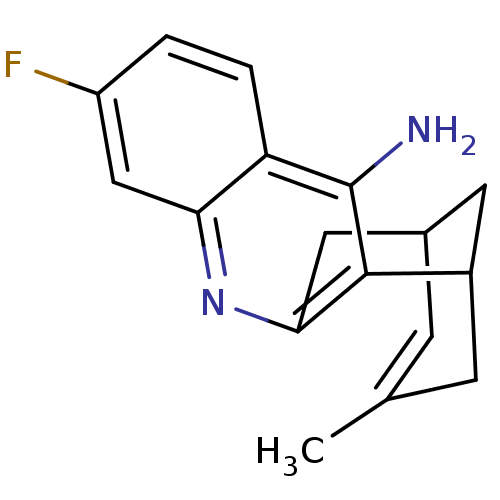
((+)-7-fluoro-15-methyl-10-azatetracyclo[11.3.1.02,...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:1,TLB:18:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H17FN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280625
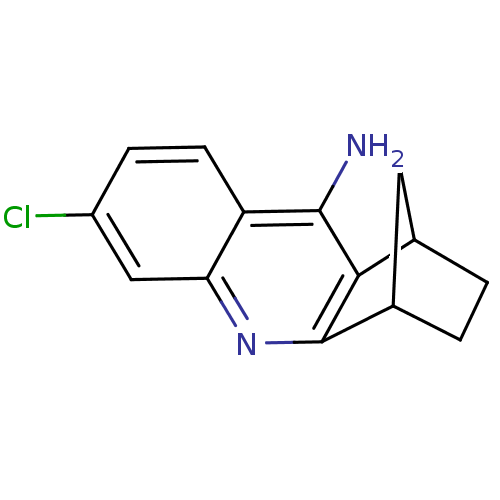
(7-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-10-11(6-9)17-14-8-2-1-7(5-8)12(14)13(10)16/h3-4,6-8H,1-2,5H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079866
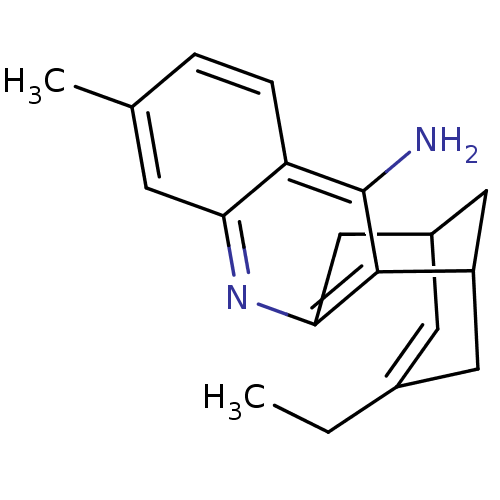
((+)-15-ethyl-7-methyl-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(C)ccc2c1N |t:2,TLB:19:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C19H22N2/c1-3-12-7-13-9-14(8-12)18-17(10-13)21-16-6-11(2)4-5-15(16)19(18)20/h4-7,13-14H,3,8-10H2,1-2H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50094626

((+)-15-ethyl-7-fluoro-10-azatetracyclo[11.3.1.02,1...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2cc(F)ccc2c1N |t:2,TLB:19:8:2.3.7:5| Show InChI InChI=1S/C18H19FN2/c1-2-10-5-11-7-12(6-10)17-16(8-11)21-15-9-13(19)3-4-14(15)18(17)20/h3-5,9,11-12H,2,6-8H2,1H3,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279984

(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079863
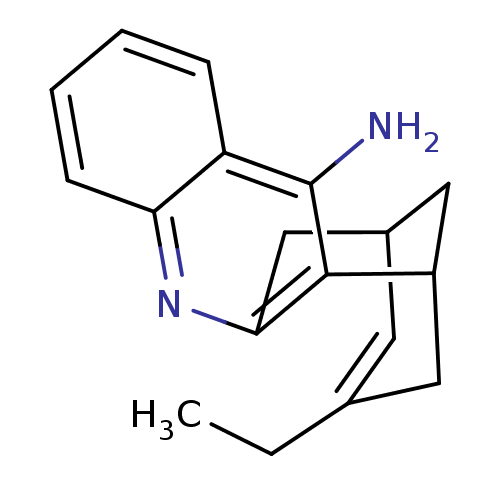
((+)-15-ethyl-10-azatetracyclo[11.3.1.02,11.04,9]he...)Show SMILES CCC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:2,TLB:18:8:2.3.7:5,THB:1:2:8.9.10:5| Show InChI InChI=1S/C18H20N2/c1-2-11-7-12-9-13(8-11)17-16(10-12)20-15-6-4-3-5-14(15)18(17)19/h3-7,12-13H,2,8-10H2,1H3,(H2,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8989
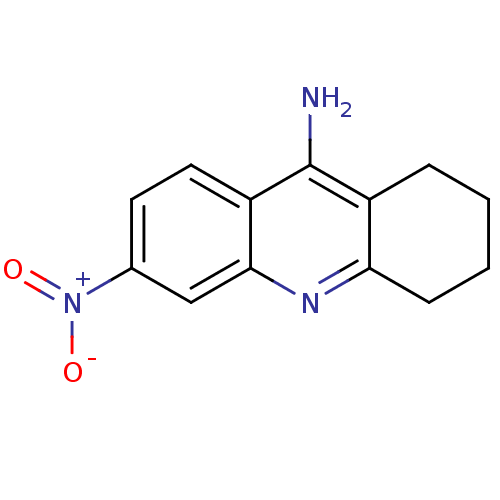
(6-nitro-1,2,3,4-tetrahydroacridin-9-amine | Tacrin...)Show InChI InChI=1S/C13H13N3O2/c14-13-9-3-1-2-4-11(9)15-12-7-8(16(17)18)5-6-10(12)13/h5-7H,1-4H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422629
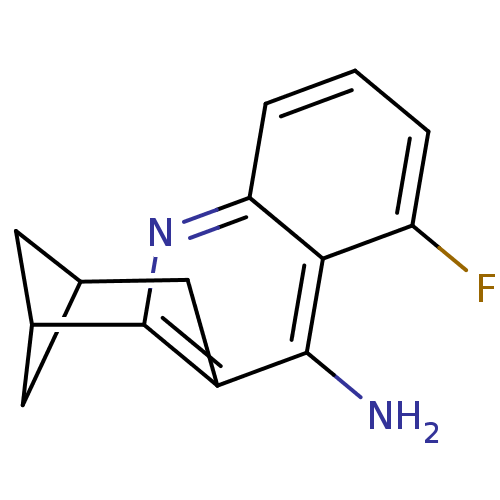
(CHEMBL1161714)Show SMILES Nc1c2CC3CC(C3)c2nc2cccc(F)c12 |(-.02,2.72,;-.02,1.18,;1.31,.41,;2.67,1.21,;4,.44,;2.67,-.36,;2.67,-1.9,;4,-1.13,;1.31,-1.13,;,-1.9,;-1.35,-1.13,;-2.68,-1.91,;-4.01,-1.14,;-4.01,.4,;-2.68,1.18,;-2.68,2.72,;-1.35,.41,)| Show InChI InChI=1S/C14H13FN2/c15-10-2-1-3-11-12(10)13(16)9-6-7-4-8(5-7)14(9)17-11/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50060470
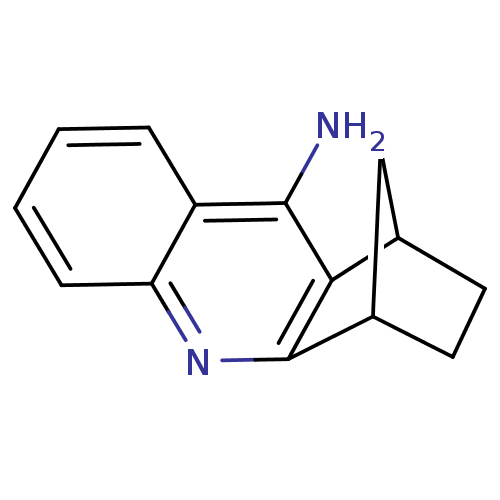
(10-azatetracyclo[10.2.1.02,11.04,9]pentadeca-2,4,6...)Show InChI InChI=1S/C14H14N2/c15-13-10-3-1-2-4-11(10)16-14-9-6-5-8(7-9)12(13)14/h1-4,8-9H,5-7H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280627
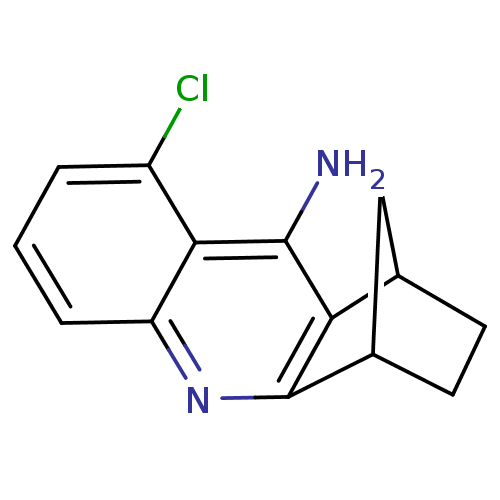
(5-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-2-1-3-10-12(9)13(16)11-7-4-5-8(6-7)14(11)17-10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50079865
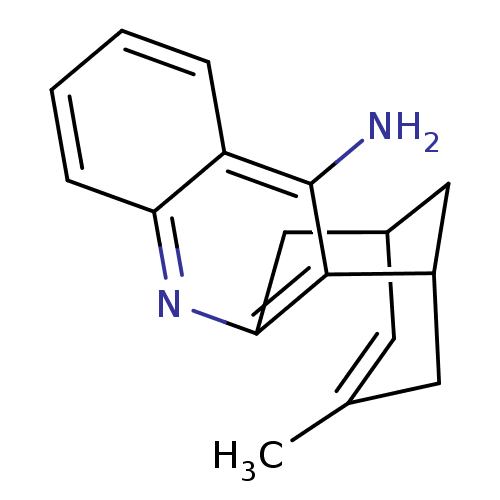
((+)-15-methyl-10-azatetracyclo[11.3.1.02,11.04,9]h...)Show SMILES CC1=CC2CC(C1)c1c(C2)nc2ccccc2c1N |t:1,TLB:17:7:1.2.6:4,THB:0:1:7.8.9:4| Show InChI InChI=1S/C17H18N2/c1-10-6-11-8-12(7-10)16-15(9-11)19-14-5-3-2-4-13(14)17(16)18/h2-6,11-12H,7-9H2,1H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against bovine acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280623
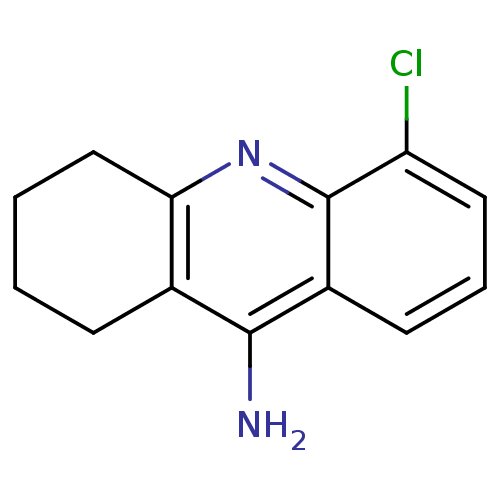
(5-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13ClN2/c14-10-6-3-5-9-12(15)8-4-1-2-7-11(8)16-13(9)10/h3,5-6H,1-2,4,7H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279995
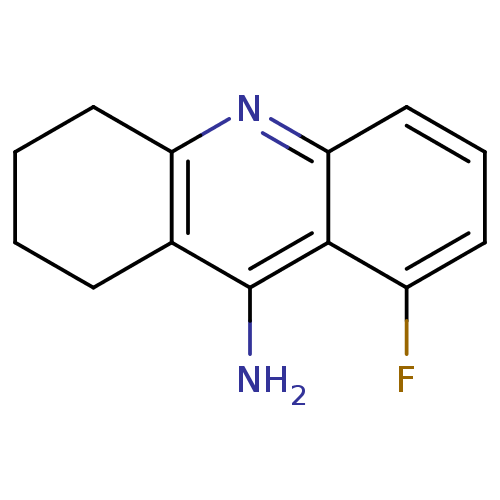
(8-Fluoro-1,2,3,4-tetrahydro-acridin-9-ylamine | 9-...)Show InChI InChI=1S/C13H13FN2/c14-9-5-3-7-11-12(9)13(15)8-4-1-2-6-10(8)16-11/h3,5,7H,1-2,4,6H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280626
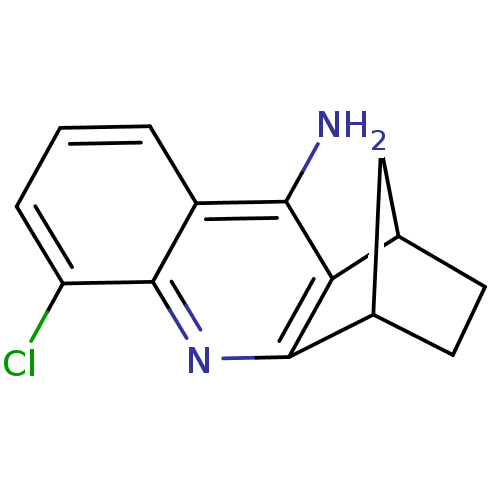
(8-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-10-3-1-2-9-12(16)11-7-4-5-8(6-7)13(11)17-14(9)10/h1-3,7-8H,4-6H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8992
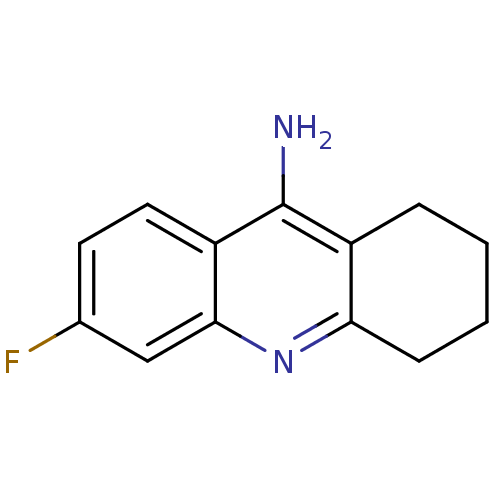
(6-fluoro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C13H13FN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8985
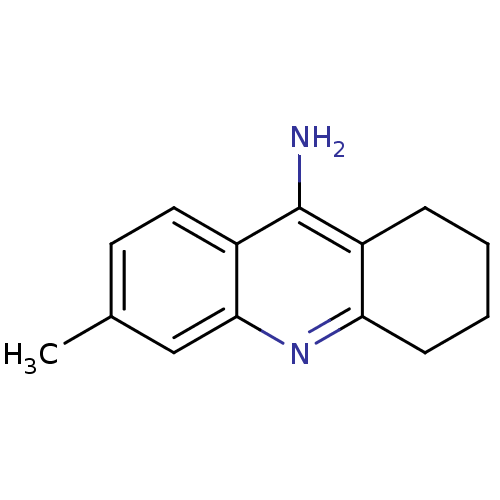
(6-methyl-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C14H16N2/c1-9-6-7-11-13(8-9)16-12-5-3-2-4-10(12)14(11)15/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422632
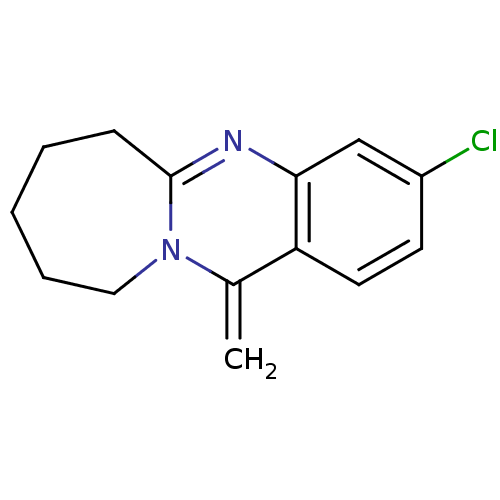
(CHEMBL1161717)Show InChI InChI=1S/C14H15ClN2/c1-10-12-7-6-11(15)9-13(12)16-14-5-3-2-4-8-17(10)14/h6-7,9H,1-5,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422630
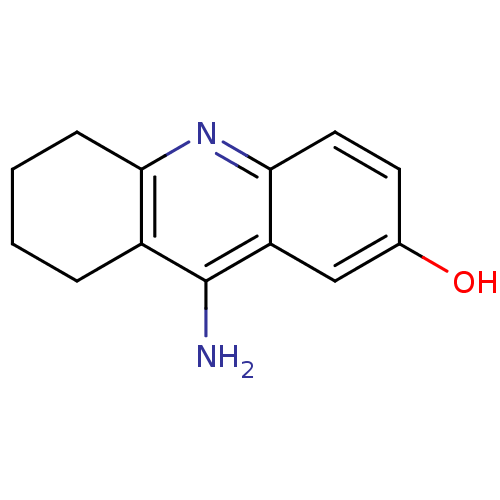
(CHEMBL1161715)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-6-5-8(16)7-10(12)13/h5-7,16H,1-4H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279988
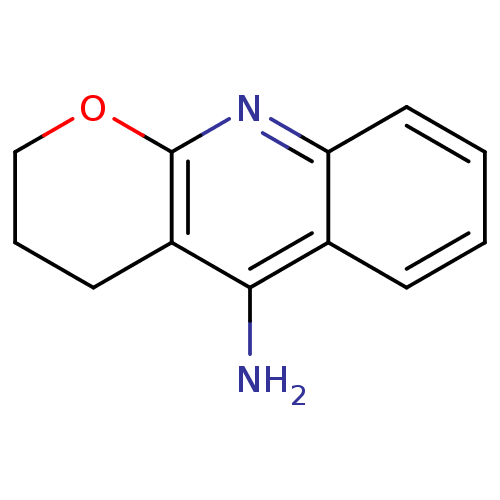
(10-Amino-3,4-dihydro-2H-1-oxa-9-azonia-anthracene ...)Show InChI InChI=1S/C12H12N2O/c13-11-8-4-1-2-6-10(8)14-12-9(11)5-3-7-15-12/h1-2,4,6H,3,5,7H2,(H2,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8990
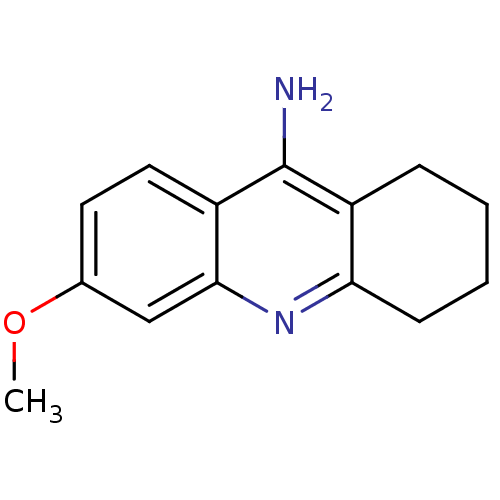
(6-methoxy-1,2,3,4-tetrahydroacridin-9-amine | Tacr...)Show InChI InChI=1S/C14H16N2O/c1-17-9-6-7-11-13(8-9)16-12-5-3-2-4-10(12)14(11)15/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422631
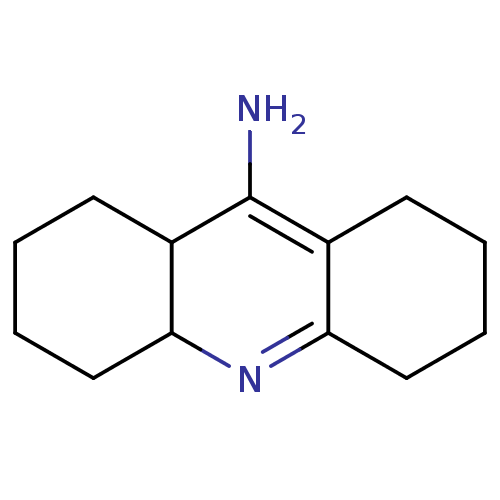
(CHEMBL1161674)Show InChI InChI=1S/C13H20N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h9,11H,1-8,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against of human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8993
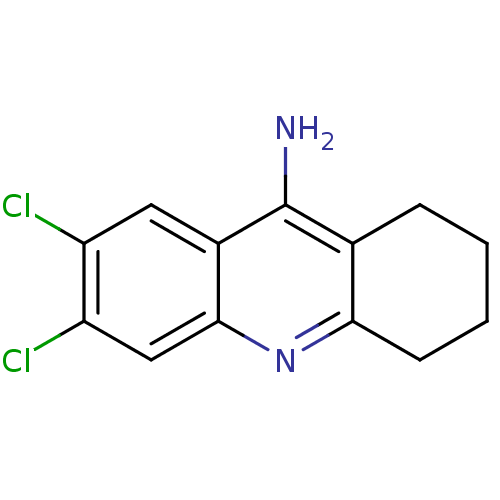
(6,7-dichloro-1,2,3,4-tetrahydroacridin-9-amine | T...)Show InChI InChI=1S/C13H12Cl2N2/c14-9-5-8-12(6-10(9)15)17-11-4-2-1-3-7(11)13(8)16/h5-6H,1-4H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422628
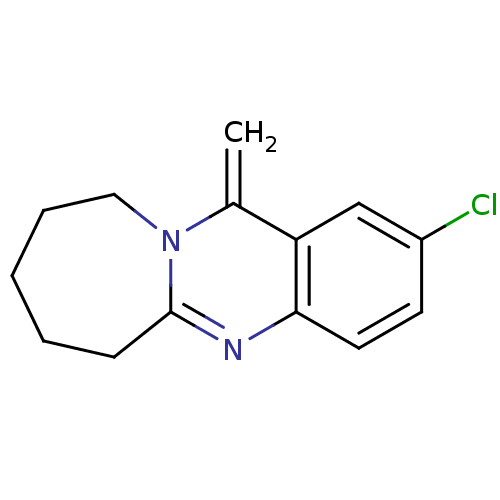
(CHEMBL1161718)Show InChI InChI=1S/C14H15ClN2/c1-10-12-9-11(15)6-7-13(12)16-14-5-3-2-4-8-17(10)14/h6-7,9H,1-5,8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8986
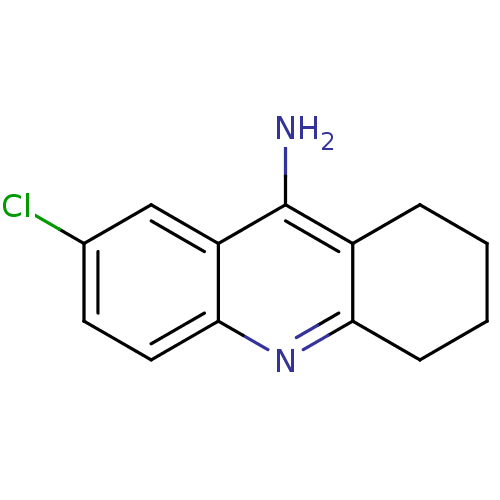
(7-chloro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-12-10(7-8)13(15)9-3-1-2-4-11(9)16-12/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279985
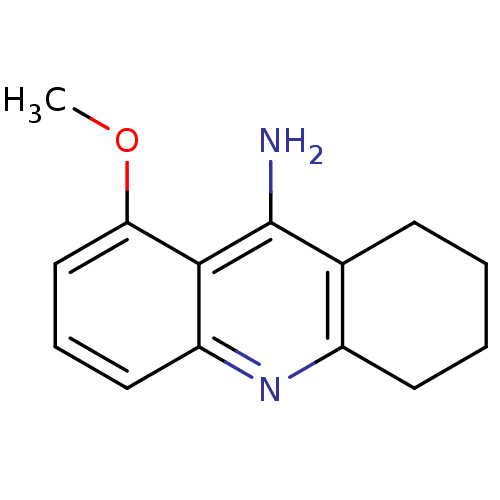
(8-Methoxy-1,2,3,4-tetrahydro-acridin-9-ylamine | 9...)Show InChI InChI=1S/C14H16N2O/c1-17-12-8-4-7-11-13(12)14(15)9-5-2-3-6-10(9)16-11/h4,7-8H,2-3,5-6H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 617 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8986

(7-chloro-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-12-10(7-8)13(15)9-3-1-2-4-11(9)16-12/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 741 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50280624
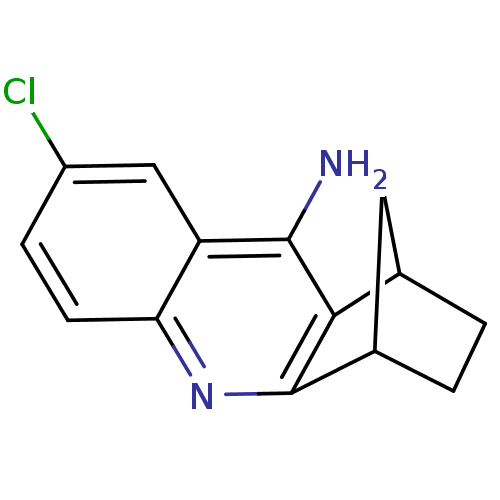
(6-chloro-10-azatetracyclo[10.2.1.02,11.04,9]pentad...)Show InChI InChI=1S/C14H13ClN2/c15-9-3-4-11-10(6-9)13(16)12-7-1-2-8(5-7)14(12)17-11/h3-4,6-8H,1-2,5H2,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422633
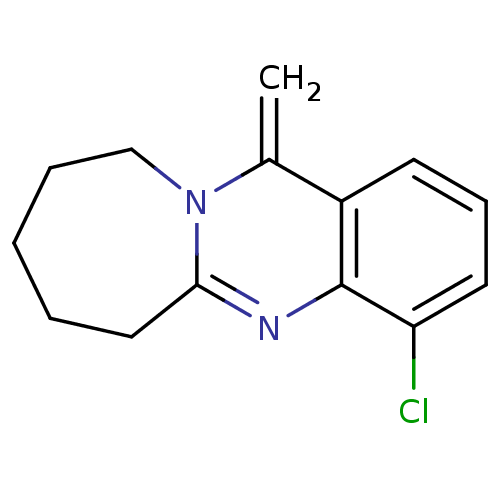
(CHEMBL1161720)Show InChI InChI=1S/C14H15ClN2/c1-10-11-6-5-7-12(15)14(11)16-13-8-3-2-4-9-17(10)13/h5-7H,1-4,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422634
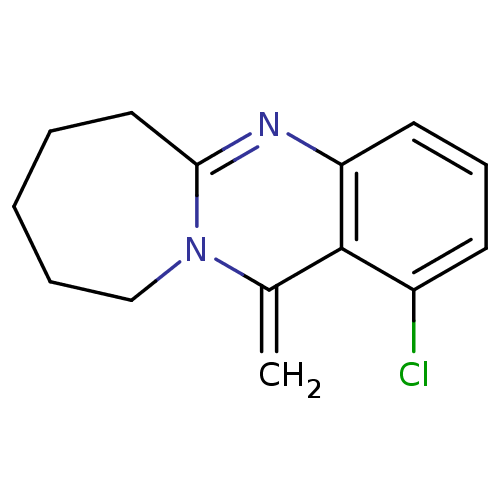
(CHEMBL1161716)Show InChI InChI=1S/C14H15ClN2/c1-10-14-11(15)6-5-7-12(14)16-13-8-3-2-4-9-17(10)13/h5-7H,1-4,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8988
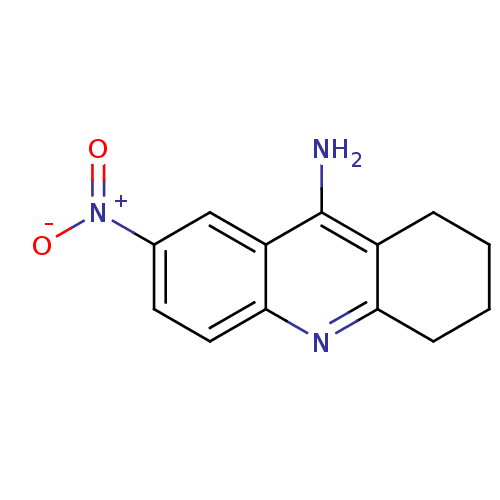
(7-nitro-1,2,3,4-tetrahydroacridin-9-amine | Tacrin...)Show InChI InChI=1S/C13H13N3O2/c14-13-9-3-1-2-4-11(9)15-12-6-5-8(16(17)18)7-10(12)13/h5-7H,1-4H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50422627
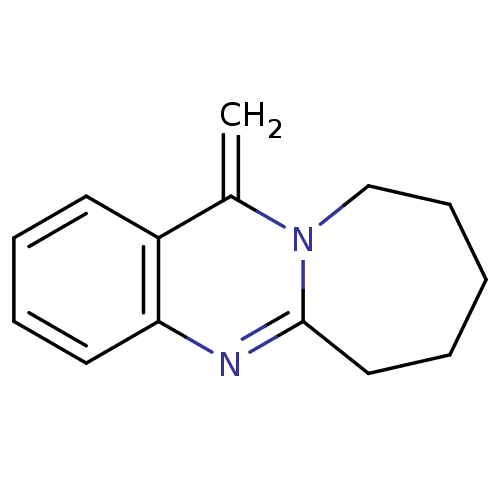
(CHEMBL1161719)Show InChI InChI=1S/C14H16N2/c1-11-12-7-4-5-8-13(12)15-14-9-3-2-6-10-16(11)14/h4-5,7-8H,1-3,6,9-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8991
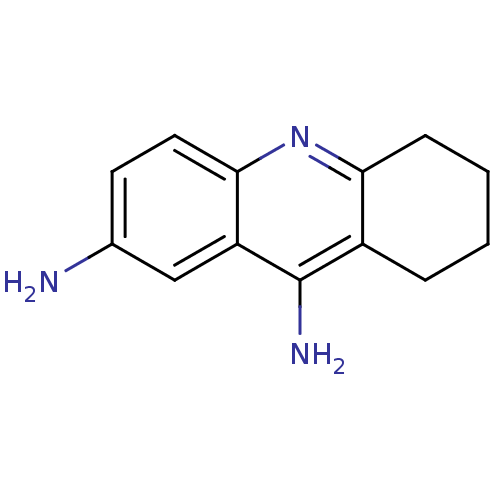
(5,6,7,8-tetrahydroacridine-2,9-diamine | 7,9-Diami...)Show InChI InChI=1S/C13H15N3/c14-8-5-6-12-10(7-8)13(15)9-3-1-2-4-11(9)16-12/h5-7H,1-4,14H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8994
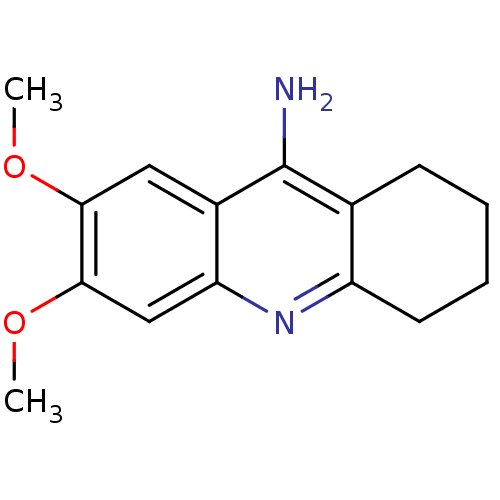
(6,7-dimethoxy-1,2,3,4-tetrahydroacridin-9-amine | ...)Show InChI InChI=1S/C15H18N2O2/c1-18-13-7-10-12(8-14(13)19-2)17-11-6-4-3-5-9(11)15(10)16/h7-8H,3-6H2,1-2H3,(H2,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279991
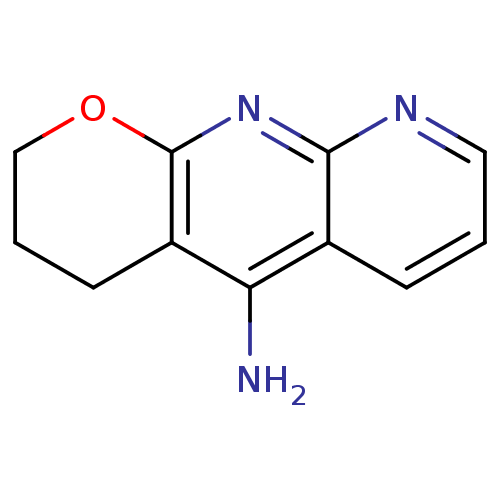
(10-Amino-3,4-dihydro-2H-1-oxa-8-aza-9-azonia-anthr...)Show InChI InChI=1S/C11H11N3O/c12-9-7-3-1-5-13-10(7)14-11-8(9)4-2-6-15-11/h1,3,5H,2,4,6H2,(H2,12,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8984
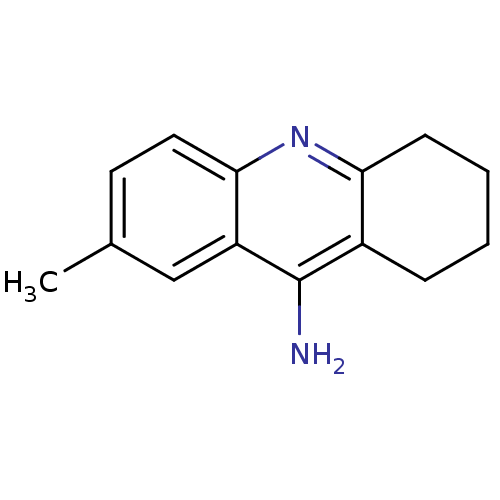
(7-methyl-1,2,3,4-tetrahydroacridin-9-amine | Tacri...)Show InChI InChI=1S/C14H16N2/c1-9-6-7-13-11(8-9)14(15)10-4-2-3-5-12(10)16-13/h6-8H,2-5H2,1H3,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50279989
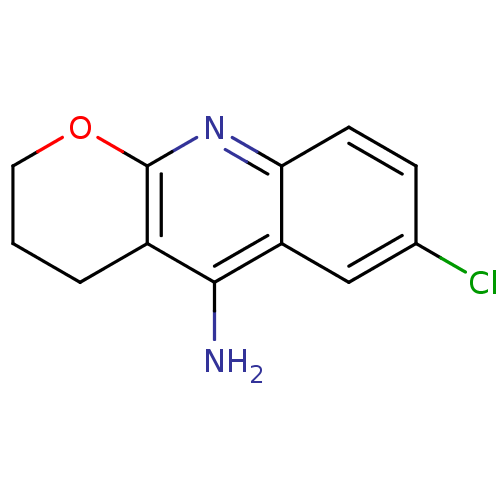
(10-Amino-6-chloro-3,4-dihydro-2H-1-oxa-9-azonia-an...)Show InChI InChI=1S/C12H11ClN2O/c13-7-3-4-10-9(6-7)11(14)8-2-1-5-16-12(8)15-10/h3-4,6H,1-2,5H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
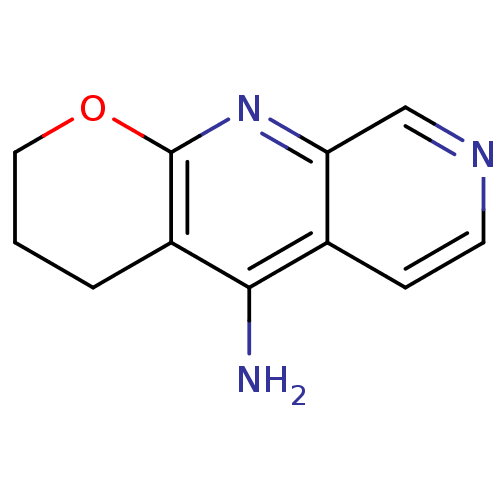
(Homo sapiens (Human)) | BDBM50279990
(10-Amino-3,4-dihydro-2H-1-oxa-7-aza-9-azonia-anthr...)Show InChI InChI=1S/C11H11N3O/c12-10-7-3-4-13-6-9(7)14-11-8(10)2-1-5-15-11/h3-4,6H,1-2,5H2,(H2,12,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona
Curated by ChEMBL
| Assay Description
Inhibitory activity against human erythrocyte acetylcholinesterase |
J Med Chem 47: 4471-82 (2004)
Article DOI: 10.1021/jm049877p
BindingDB Entry DOI: 10.7270/Q26974WJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data