 Found 14 hits with Last Name = 'martinez' and Initial = 'ej'
Found 14 hits with Last Name = 'martinez' and Initial = 'ej' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50001750
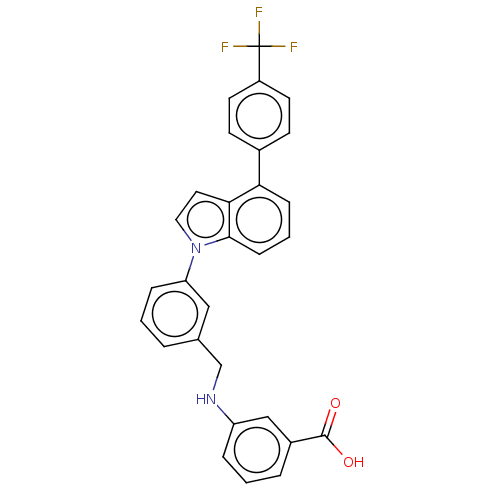
(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of methyl-transferase activity in GST-tagged recombinant SET7/9 (unknown origin) expressed in Escherichia coli BL21 using H3(1-20)-cys-bio... |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50001750

(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of 10 nM SET7 (unknown origin)-mediated methylation of histone H3(1-20)cys-biotin at 15 to 50 uM after 5 mins by scintillation proximity a... |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50001750

(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of 100 nM SET7 (unknown origin)-mediated methylation of histone H3(1-20)cys-biotin at 15 to 50 uM after 5 mins by scintillation proximity ... |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50001750

(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of 50 nM SET7 (unknown origin)-mediated methylation of histone H3(1-20)cys-biotin at 15 to 50 uM after 5 mins by scintillation proximity a... |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50001750

(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of SET7 (unknown origin)-mediated methylation of histone H3(1-20)cys-biotin after 5 mins by scintillation proximity assay in presence of [... |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50001750

(CHEMBL3238424)Show SMILES OC(=O)c1cccc(NCc2cccc(c2)-n2ccc3c(cccc23)-c2ccc(cc2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21F3N2O2/c30-29(31,32)22-12-10-20(11-13-22)25-8-3-9-27-26(25)14-15-34(27)24-7-1-4-19(16-24)18-33-23-6-2-5-21(17-23)28(35)36/h1-17,33H,18H2,(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Rockefeller University
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal GST-tagged human G9a-mediated-methylation of histone3 expressed in Escherichia coli BL21(DE3) by MALDI-TOF mass spectrometry |
Bioorg Med Chem 22: 2253-60 (2014)
Article DOI: 10.1016/j.bmc.2014.02.024
BindingDB Entry DOI: 10.7270/Q2FB54FG |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM26066
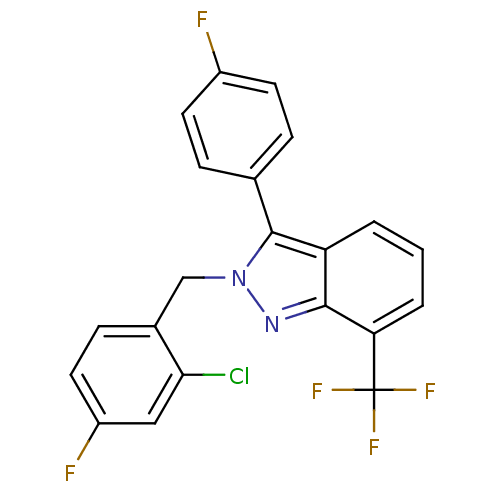
(2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...)Show SMILES Fc1ccc(cc1)-c1n(Cc2ccc(F)cc2Cl)nc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C21H12ClF5N2/c22-18-10-15(24)9-6-13(18)11-29-20(12-4-7-14(23)8-5-12)16-2-1-3-17(19(16)28-29)21(25,26)27/h1-10H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 179 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19993

(CHEMBL62136 | N-[4-(1,1,1,3,3,3-hexafluoro-2-hydro...)Show SMILES OC(c1ccc(cc1)N(CC(F)(F)F)S(=O)(=O)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H12F9NO3S/c18-14(19,20)10-27(31(29,30)13-4-2-1-3-5-13)12-8-6-11(7-9-12)15(28,16(21,22)23)17(24,25)26/h1-9,28H,10H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM426493
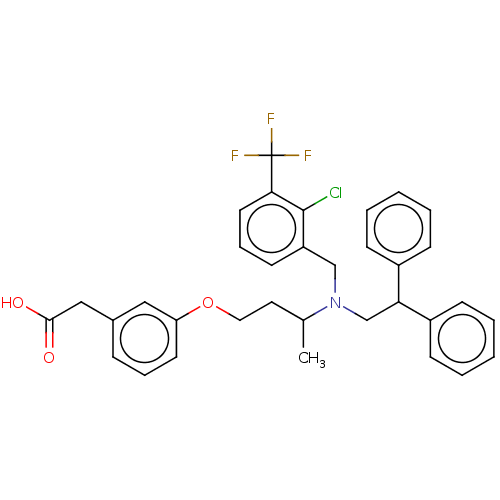
(US10543183, Compound SB742881 | US10669296, Compou...)Show SMILES CC(CCOc1cccc(CC(O)=O)c1)N(CC(c1ccccc1)c1ccccc1)Cc1cccc(c1Cl)C(F)(F)F Show InChI InChI=1S/C34H33ClF3NO3/c1-24(18-19-42-29-16-8-10-25(20-29)21-32(40)41)39(22-28-15-9-17-31(33(28)35)34(36,37)38)23-30(26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-17,20,24,30H,18-19,21-23H2,1H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM426493

(US10543183, Compound SB742881 | US10669296, Compou...)Show SMILES CC(CCOc1cccc(CC(O)=O)c1)N(CC(c1ccccc1)c1ccccc1)Cc1cccc(c1Cl)C(F)(F)F Show InChI InChI=1S/C34H33ClF3NO3/c1-24(18-19-42-29-16-8-10-25(20-29)21-32(40)41)39(22-28-15-9-17-31(33(28)35)34(36,37)38)23-30(26-11-4-2-5-12-26)27-13-6-3-7-14-27/h2-17,20,24,30H,18-19,21-23H2,1H3,(H,40,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM26066

(2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...)Show SMILES Fc1ccc(cc1)-c1n(Cc2ccc(F)cc2Cl)nc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C21H12ClF5N2/c22-18-10-15(24)9-6-13(18)11-29-20(12-4-7-14(23)8-5-12)16-2-1-3-17(19(16)28-29)21(25,26)27/h1-10H,11H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM19992

(2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...)Show SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1 Show InChI InChI=1S/C33H31ClF3NO3/c34-32-27(15-8-17-30(32)33(35,36)37)22-38(18-9-19-41-28-16-7-10-24(20-28)21-31(39)40)23-29(25-11-3-1-4-12-25)26-13-5-2-6-14-26/h1-8,10-17,20,29H,9,18-19,21-23H2,(H,39,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Rgenix, Inc.
US Patent
| Assay Description
TBD |
US Patent US10669296 (2020)
BindingDB Entry DOI: 10.7270/Q2V127W1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data