

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528011 (US11179399, Example 44_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527982 (US11179399, Example 25_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528009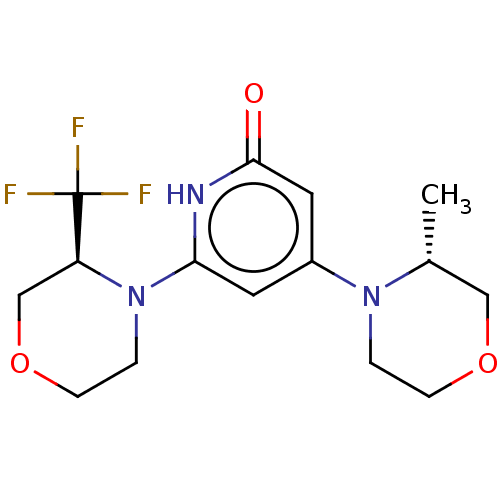 (US11179399, Example 43_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528013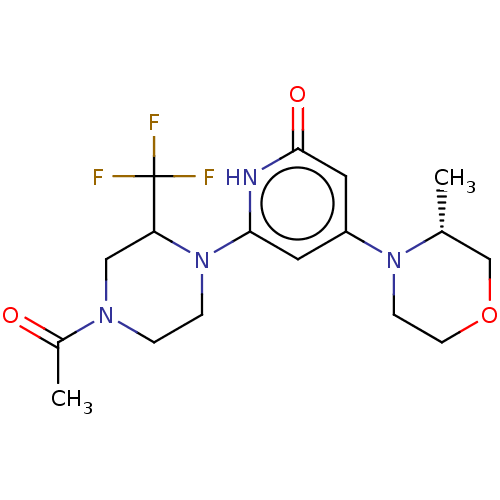 (6-[4-acetyl-2-(trifluoromethyl)piperazin-1-yl]-4-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527937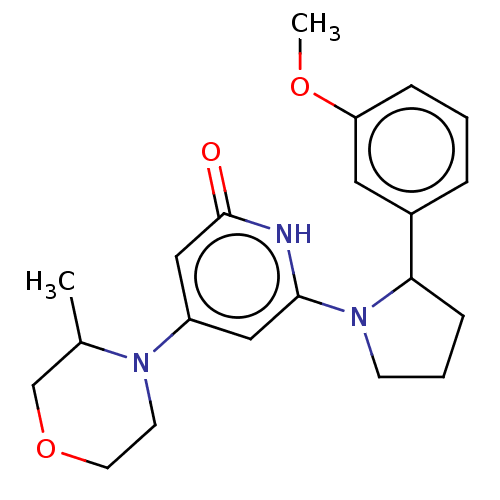 (6-[2-(3-methoxyphenyl)pyrrolidin-1-yl]-4-(3-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527981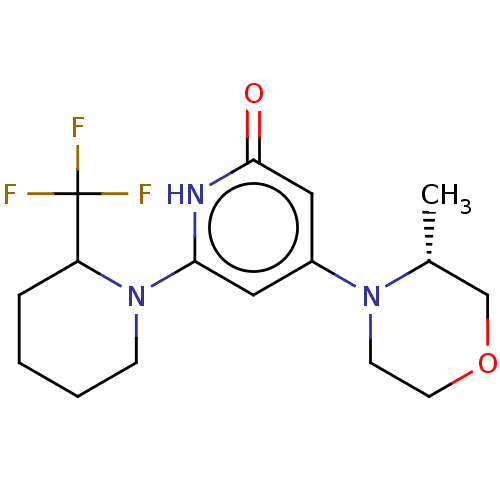 ((R) and (S) 4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528014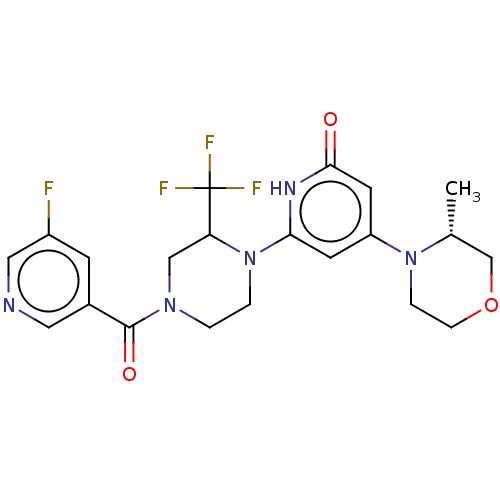 (6-[4-(5-fluoropyridine-3-carbonyl)-2-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528012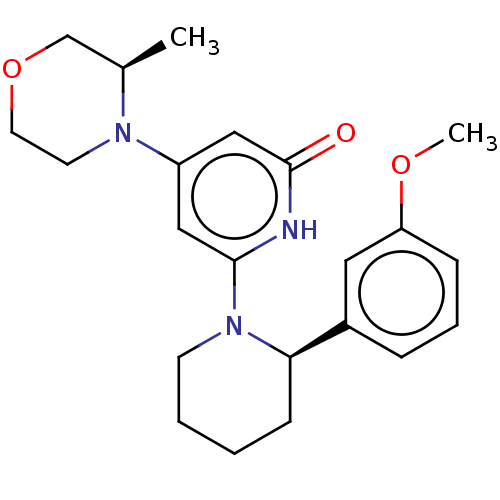 (US11179399, Example 44_2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528015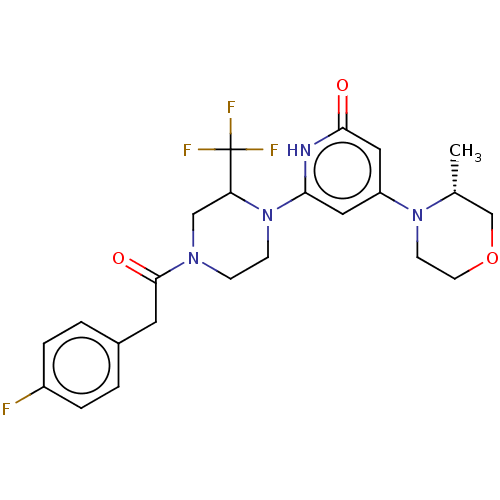 (6-[4-[2-(4-fluorophenyl)acetyl]-2-(trifluoromethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527961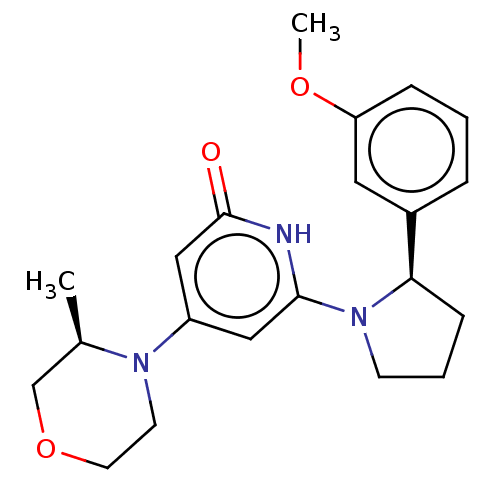 (US11179399, Example 17_2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527992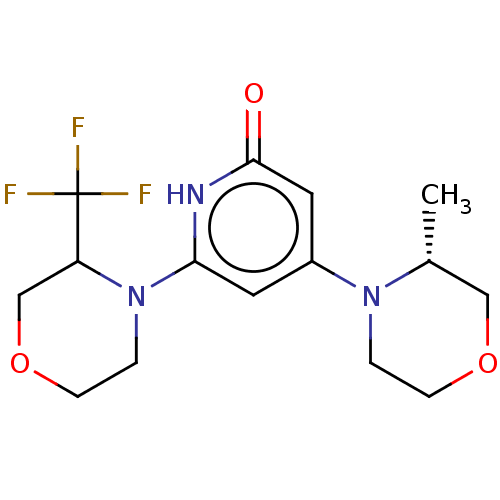 (4-[(3R)-3-methylmorpholin-4-yl]-6-[3-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510506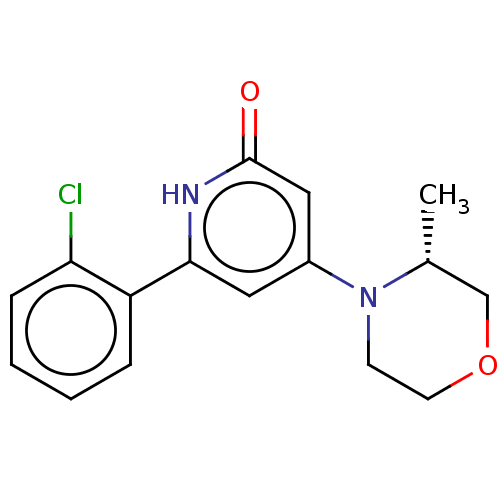 (6-(2-chlorophenyl)-4-[(3R)-3-methylmorpholin-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510507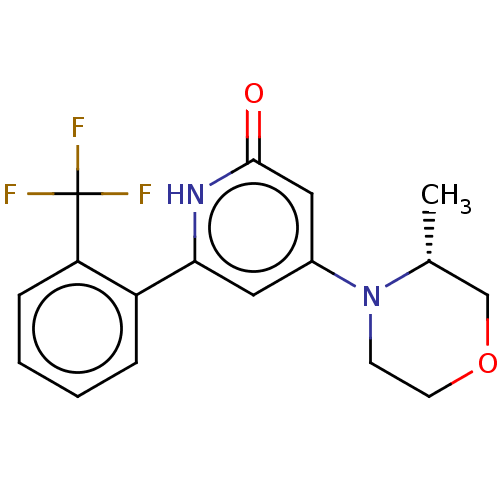 (4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510506 (6-(2-chlorophenyl)-4-[(3R)-3-methylmorpholin-4-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510507 (4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527990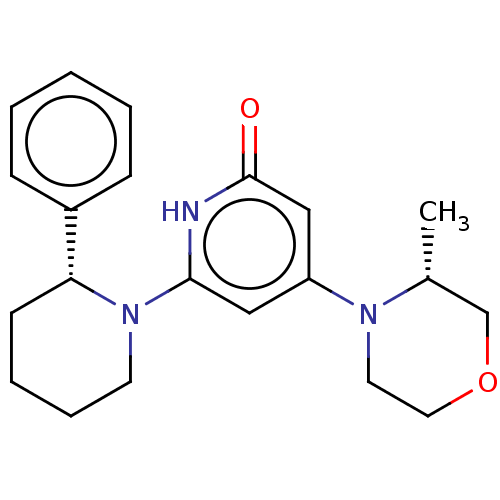 (4-[(3R)-3-methylmorpholin-4-yl]-6-[(2R)-2-phenyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527995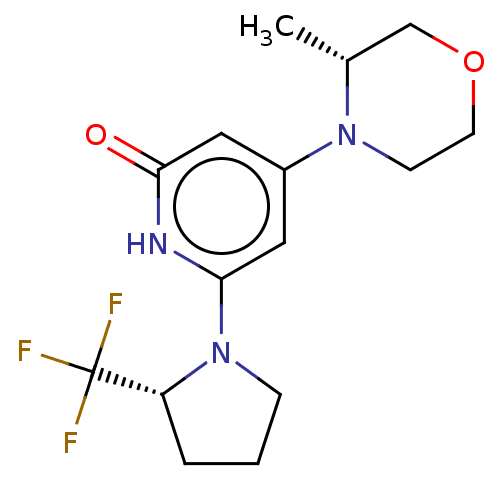 (4-[(3R)-3-methylmorpholin-4-yl]-6-[(2R)-2-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528016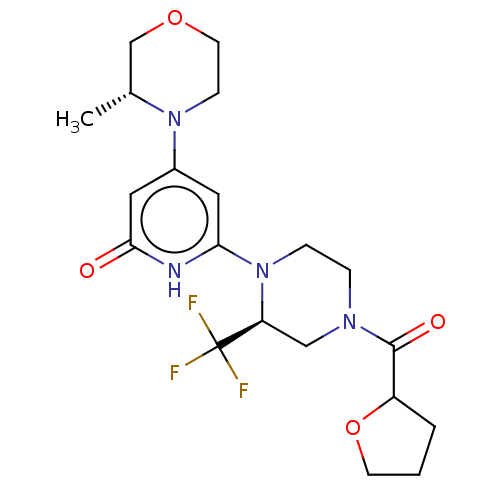 (US11179399, Example 48_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510503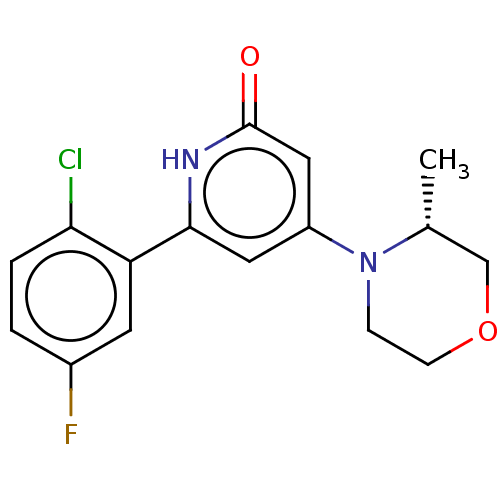 (6-(2-chloro-5-fluoro-phenyl)-4-[(3R)-3-methylmorph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510498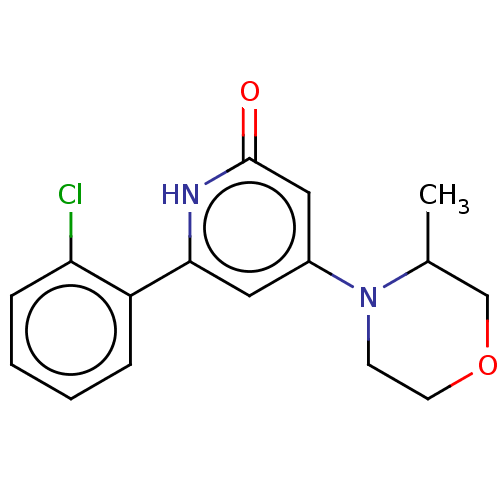 (6-(2-chlorophenyl)-4-(3-methylmorpholin-4-yl)-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510503 (6-(2-chloro-5-fluoro-phenyl)-4-[(3R)-3-methylmorph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528007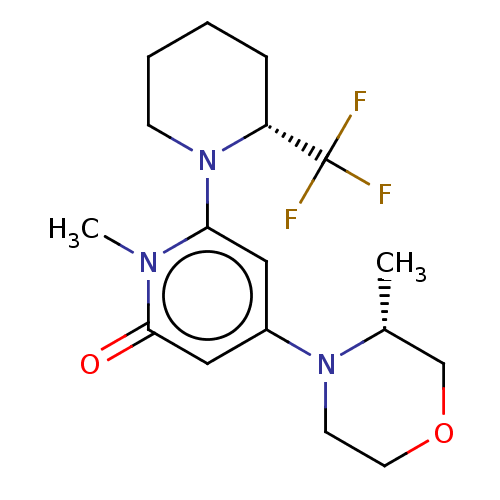 (1-methyl-4-[(3R)-3-methylmorpholin-4-yl]-6-[(2R)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527959 ((R) and (S) 6-[2-(3-methoxyphenyl)pyrrolidin-1-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527979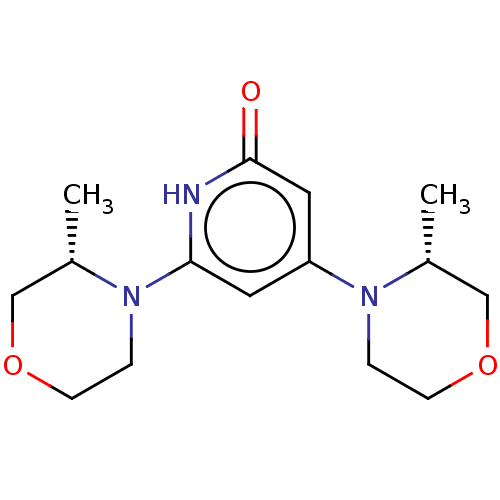 (US11179399, Example 24_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527997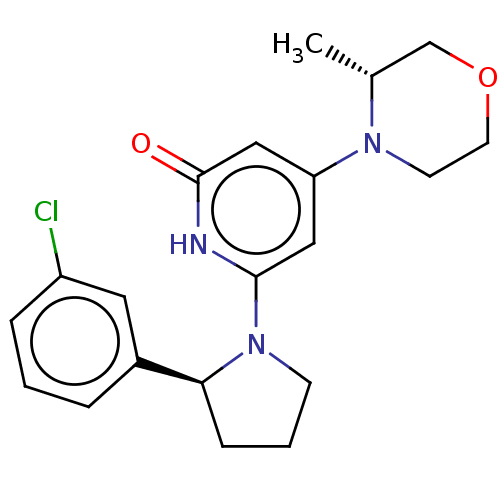 (US11179399, Example 36_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510498 (6-(2-chlorophenyl)-4-(3-methylmorpholin-4-yl)-1H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528017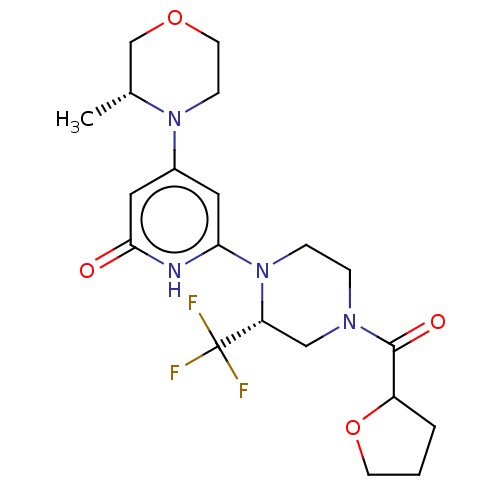 (US11179399, Example 48_2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510505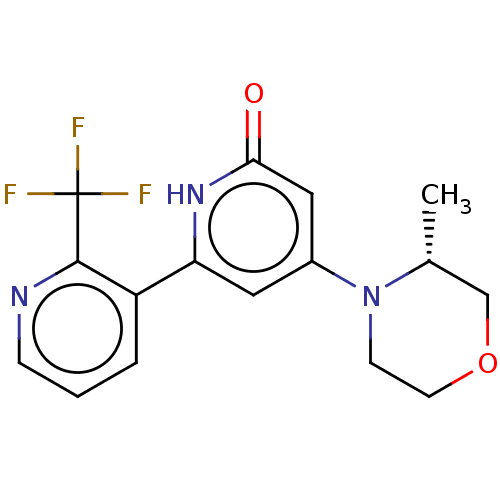 (4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM600779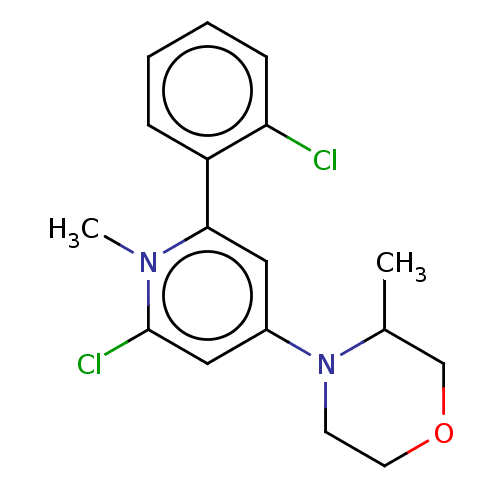 (6-(2-chlorophenyl)-1-methyl-4-(3-methylmorpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510505 (4-[(3R)-3-methylmorpholin-4-yl]-6-[2-(trifluoromet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510499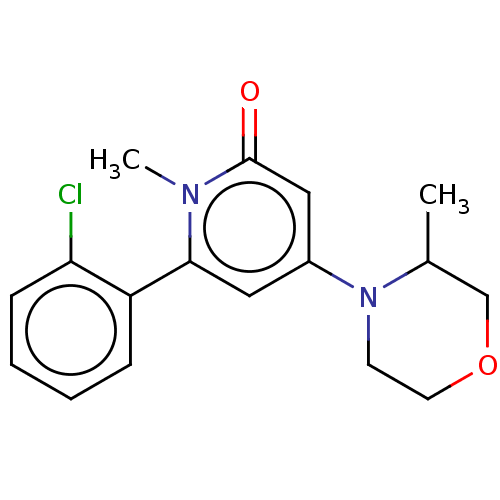 (6-(2-chlorophenyl)-1-methyl-4-(3-methylmorpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527939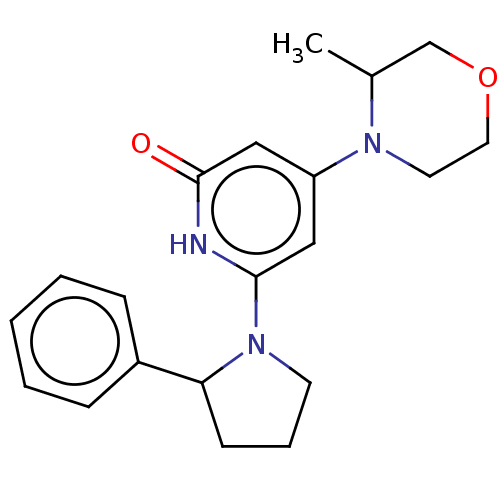 (4-(3-methylmorphol in-4-yl)-6-(2-phenylpyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527999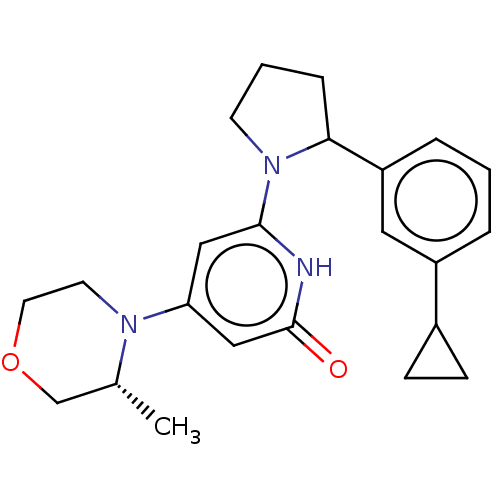 (6-[2-(3-cyclopropylphenyl)pyrrolidin-1-yl]-4-[(3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527951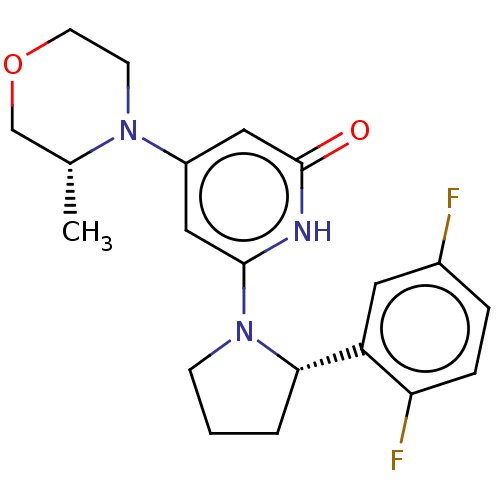 (US11179399, Example 14_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527960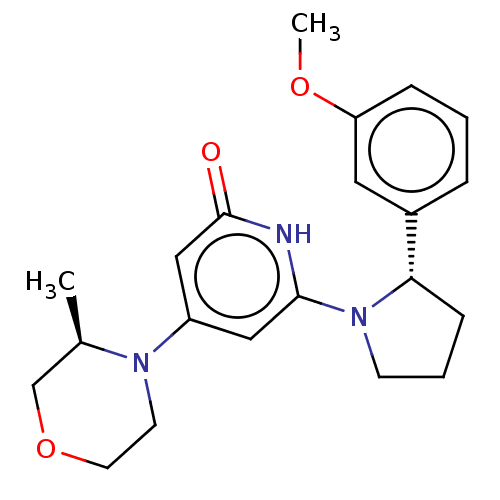 (US11179399, Example 17_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527964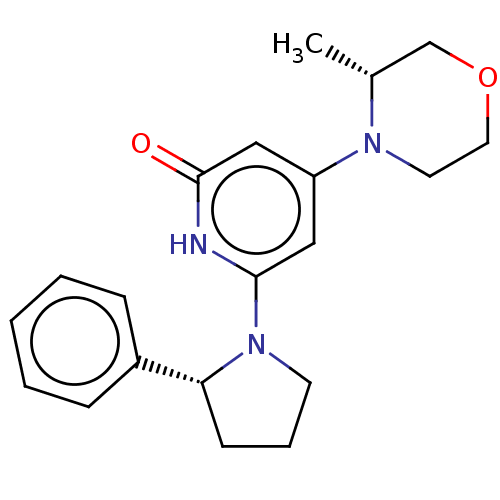 (US11179399, Example 18_2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527996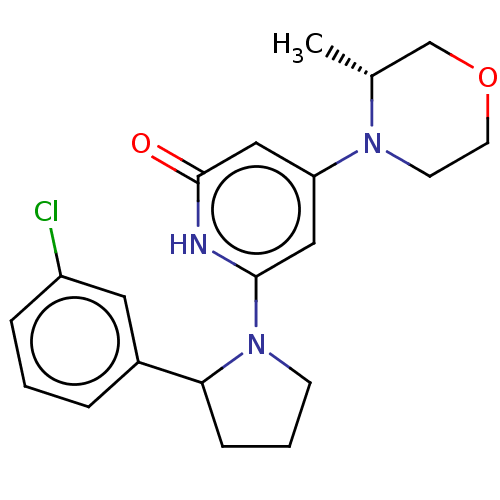 ((R) and (S) 6-[2-(3-chlorophenyl)pyrrolidin-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527998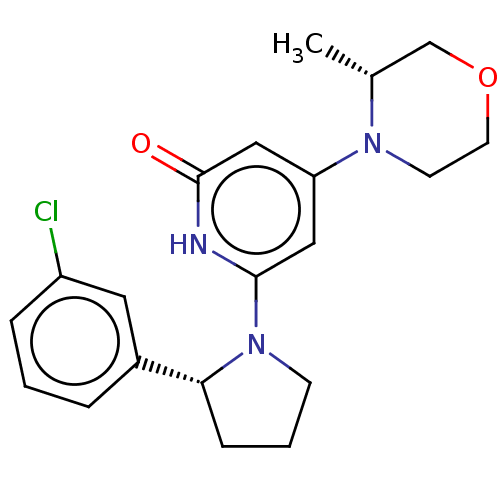 (US11179399, Example 36_2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527947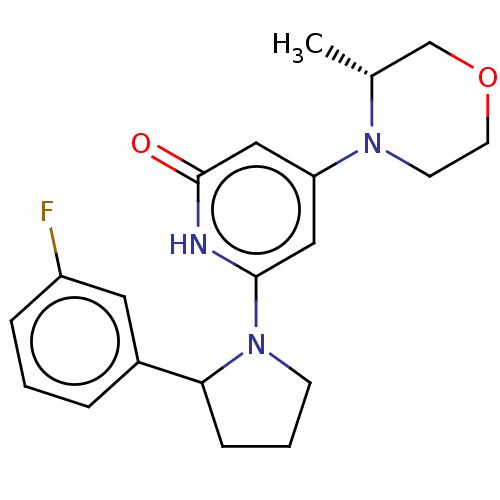 ((R) and (S) 6-[2-(3-fluorophenyl)pyrrolidin-1-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528018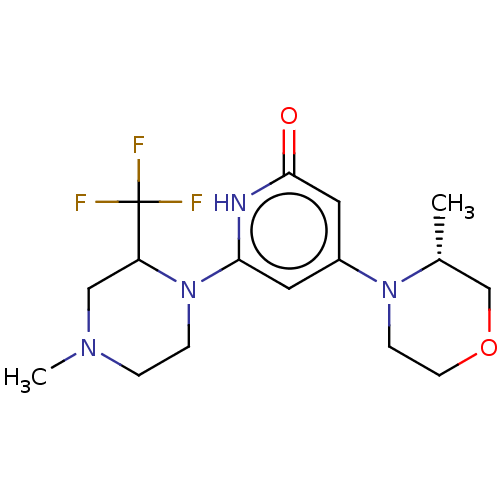 (4-[(3R)-3-methylmorpholin-4-yl]-6-[4-methyl-2-(tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510504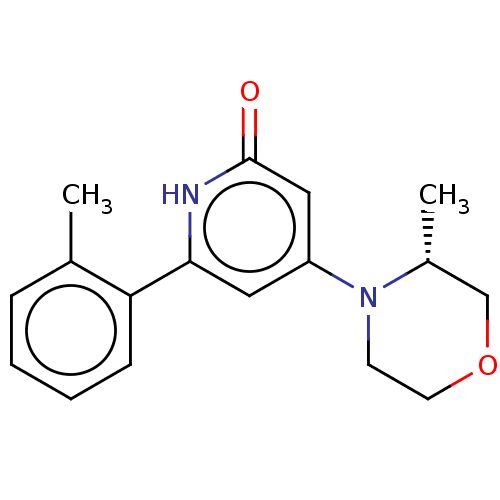 (4-[(3R)-3-methylmorpholin-4-yl]-6-(o-tolyl)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510509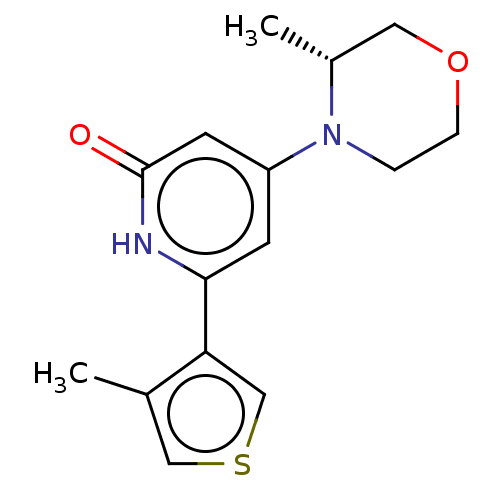 (4-[(3R)-3-methylmorpholin-4-yl]-6-(4-methyl-3-thie...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510495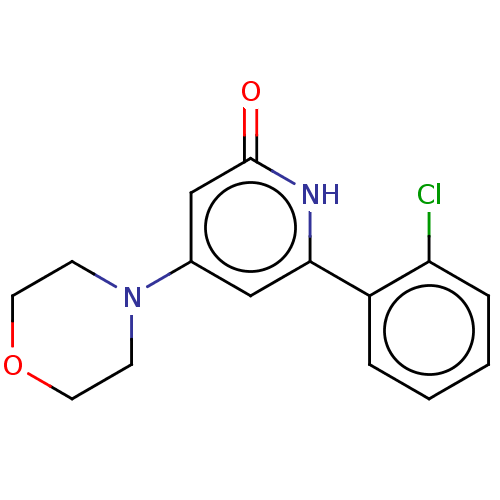 (6-(2-chlorophenyl)-4-morpholino-1H-pyridin-2-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q27948MS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (Homo sapiens (Human)) | BDBM200906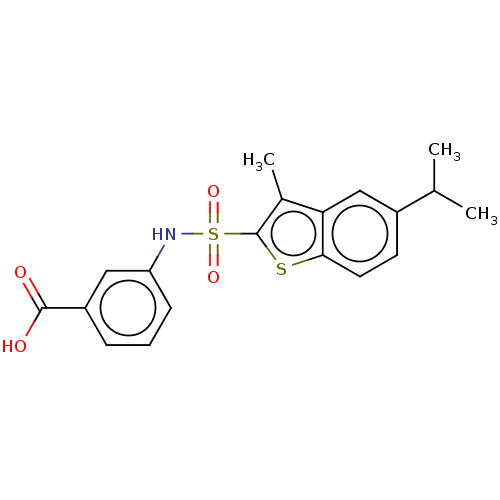 (US9233946, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
KANCERA AB US Patent | Assay Description Method A: The kinase activity of the bi-functional enzyme is readily quantified based on the production of F-2,6-P2 as described by Van Schaftingen e... | US Patent US9233946 (2016) BindingDB Entry DOI: 10.7270/Q2V123KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510504 (4-[(3R)-3-methylmorpholin-4-yl]-6-(o-tolyl)-1H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM528008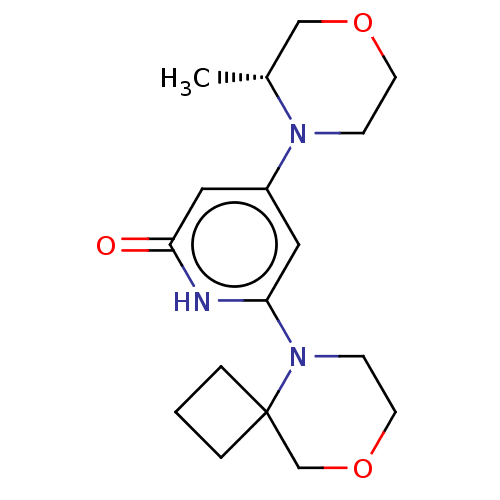 (4-[(3R)-3-methylmorpholin-4-yl]-6-(8-oxa-5-azaspir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM510495 (6-(2-chlorophenyl)-4-morpholino-1H-pyridin-2-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FB562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527957 (US11179399, Example 16_1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527977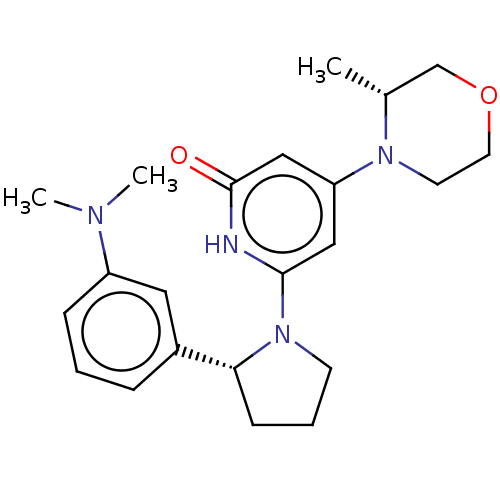 ((R) and (S) 6-[2-[3-(dimethylamino)phenyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 3-kinase catalytic subunit type 3 (Homo sapiens (Human)) | BDBM527978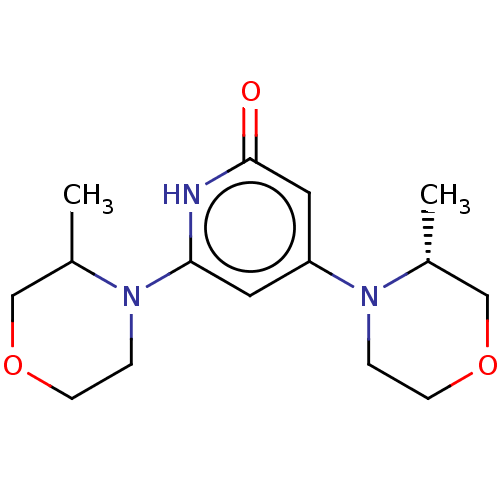 ((R) and (S) 4-[(3R)-3-methylmorpholin-4-yl]-6-(3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Dilution series of compounds of the invention were prepared in DMSO at 100 times the final assay concentration (n1=n0/3 in 10 points). The compounds ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2891926 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 272 total ) | Next | Last >> |