

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314948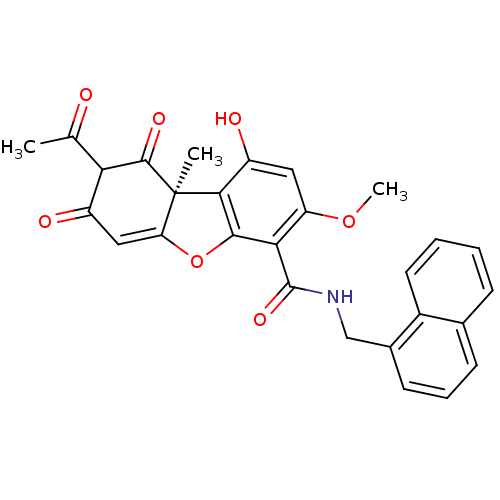 ((S)-8-Acetyl-6,9-dihydroxy-3-methoxy-6b-methyl-1,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to PPARgamma | Bioorg Med Chem Lett 20: 2095-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.073 BindingDB Entry DOI: 10.7270/Q2RX9D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pro polyprotein (Human T-cell leukemia virus 1 (strain Japan ATK-1 ...) | BDBM50155805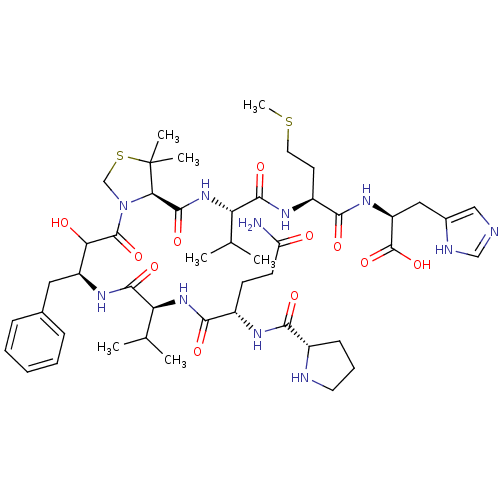 (CHEMBL415620 | H-Pro-Gln-Val-Apns-Dmt-Val-Met-His-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory binding affinity towards synthesized human T-cell leukemia virus type I (HTLV-1) protease | Bioorg Med Chem Lett 14: 5925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.034 BindingDB Entry DOI: 10.7270/Q2CC11GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pro polyprotein (Human T-cell leukemia virus 1 (strain Japan ATK-1 ...) | BDBM50155805 (CHEMBL415620 | H-Pro-Gln-Val-Apns-Dmt-Val-Met-His-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory binding affinity towards recombinant human T-cell leukemia virus type I (HTLV-1) protease | Bioorg Med Chem Lett 14: 5925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.034 BindingDB Entry DOI: 10.7270/Q2CC11GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pro polyprotein (Human T-cell leukemia virus 1 (strain Japan ATK-1 ...) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory binding affinity towards recombinant human T-cell leukemia virus type I (HTLV-1) protease | Bioorg Med Chem Lett 14: 5925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.034 BindingDB Entry DOI: 10.7270/Q2CC11GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pro polyprotein (Human T-cell leukemia virus 1 (strain Japan ATK-1 ...) | BDBM912 ((3S,4S)-3-hydroxy-4-[(2S)-2-[(3S,4S)-3-hydroxy-6-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibitory binding affinity towards synthesized human T-cell leukemia virus type I (HTLV-1) protease | Bioorg Med Chem Lett 14: 5925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.09.034 BindingDB Entry DOI: 10.7270/Q2CC11GW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50392953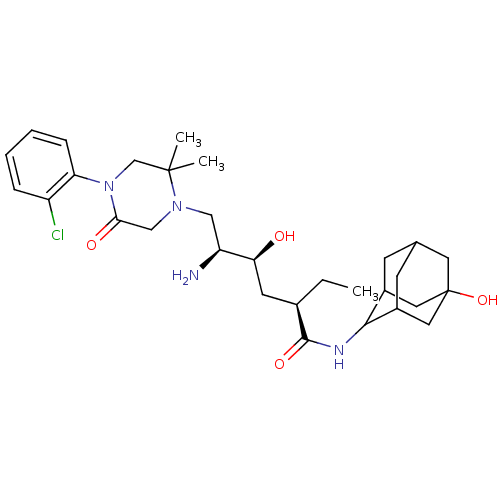 (CHEMBL2152353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin compound treated for 10 mins before substrate addition measured after 90 mins by fluorescence method | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402219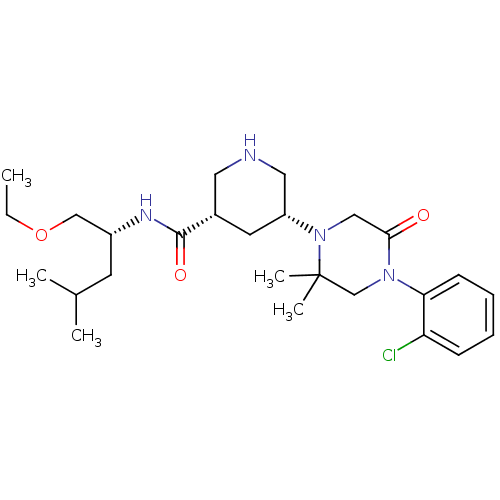 (CHEMBL2204732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402219 (CHEMBL2204732) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin compound treated for 10 mins before substrate addition measured after 90 mins by fluorescence method | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402224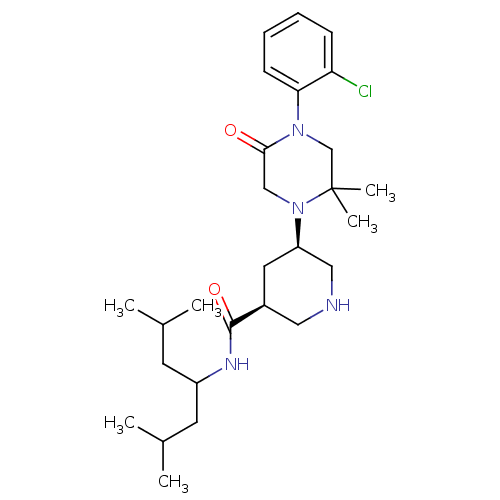 (CHEMBL2204739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50392953 (CHEMBL2152353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin activity after 60 mins by competitive RIA | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402221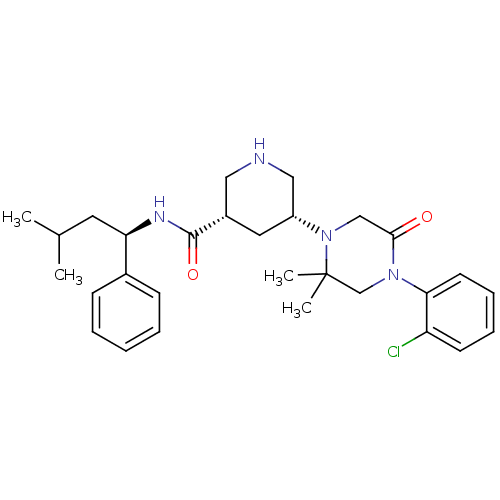 (CHEMBL2204017) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402217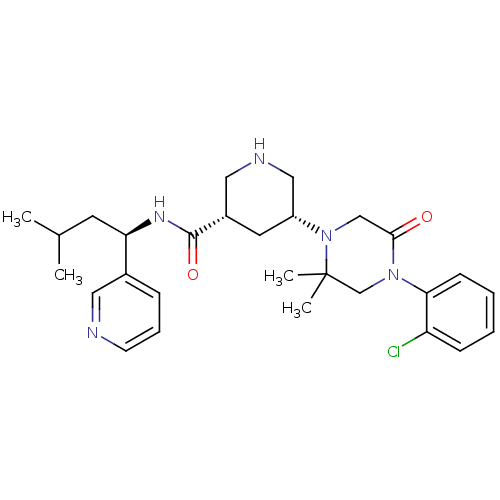 (CHEMBL2204734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402223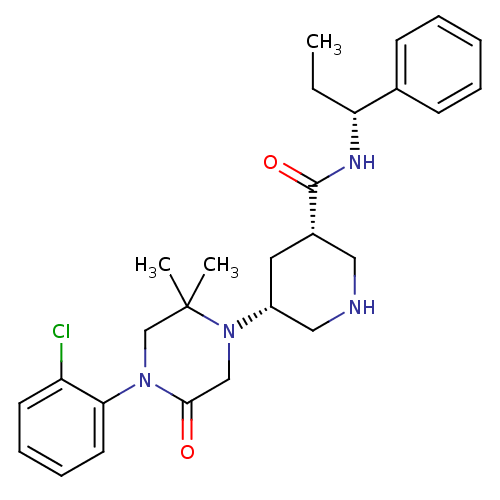 (CHEMBL2204740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402220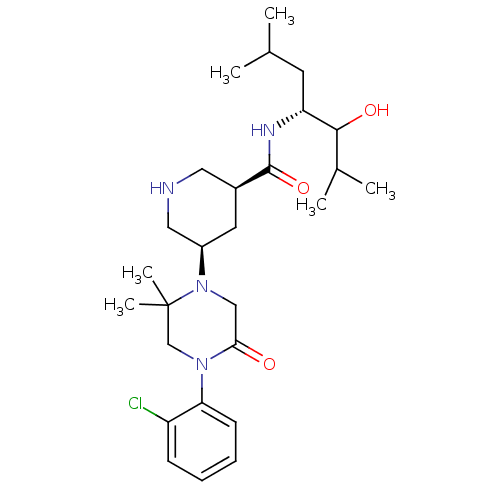 (CHEMBL2204018) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402222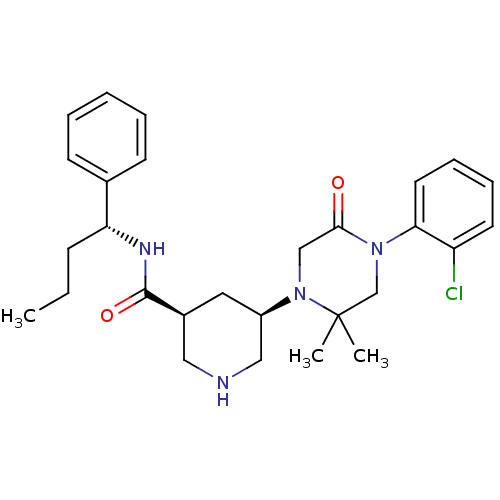 (CHEMBL2204741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402220 (CHEMBL2204018) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402225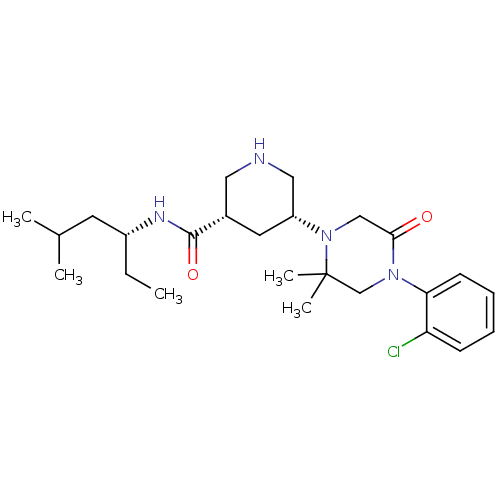 (CHEMBL2204738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human plasma renin activity after 60 mins by competitive RIA | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Macaca fascicularis) | BDBM50402217 (CHEMBL2204734) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50392953 (CHEMBL2152353) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of cynomolgus monkey plasma renin activity after 60 mins by competitive RIA | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402223 (CHEMBL2204740) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402220 (CHEMBL2204018) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402220 (CHEMBL2204018) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402226 (CHEMBL2204737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Macaca fascicularis) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of cynomolgus monkey plasma renin activity after 60 mins by competitive RIA | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402218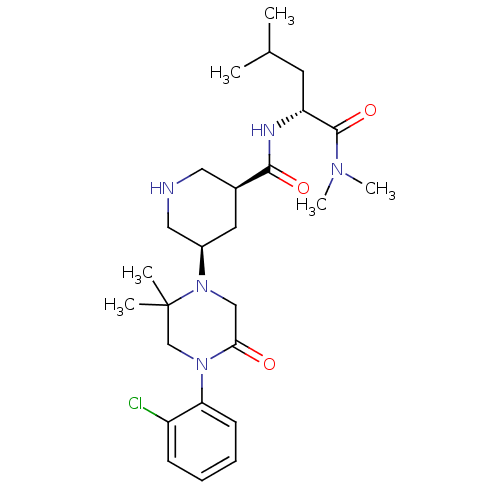 (CHEMBL2204733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402221 (CHEMBL2204017) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402224 (CHEMBL2204739) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Macaca fascicularis) | BDBM50402225 (CHEMBL2204738) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402222 (CHEMBL2204741) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402218 (CHEMBL2204733) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402226 (CHEMBL2204737) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| C-1-tetrahydrofolate synthase, cytoplasmic (Homo sapiens (Human)) | BDBM50535669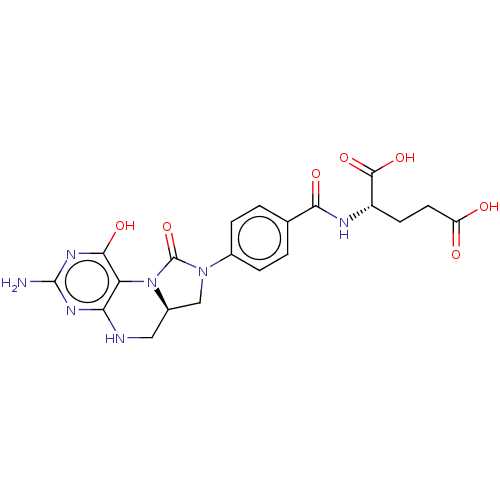 (CHEMBL1233930) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTHFD1 ( 1 to 306 residues) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before folitixorin and ... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402227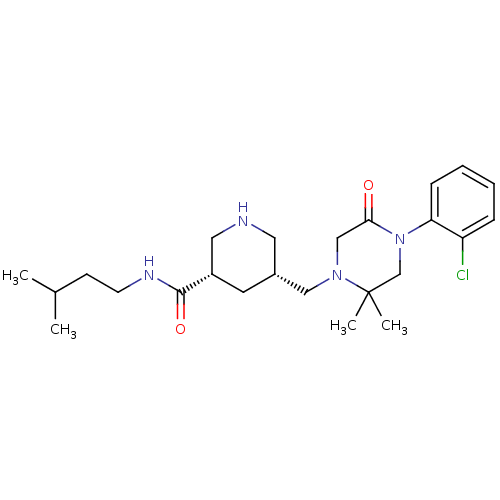 (CHEMBL2204736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50314948 ((S)-8-Acetyl-6,9-dihydroxy-3-methoxy-6b-methyl-1,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PPARgamma assessed as transcriptional activity | Bioorg Med Chem Lett 20: 2095-8 (2010) Article DOI: 10.1016/j.bmcl.2010.02.073 BindingDB Entry DOI: 10.7270/Q2RX9D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535669 (CHEMBL1233930) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 663 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of His-tagged human MTHFD2 ( 36 to 350 residues) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before folitixorin and... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535650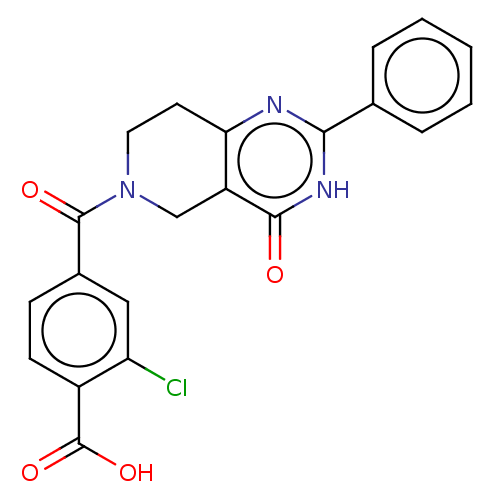 (CHEMBL4540255) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402228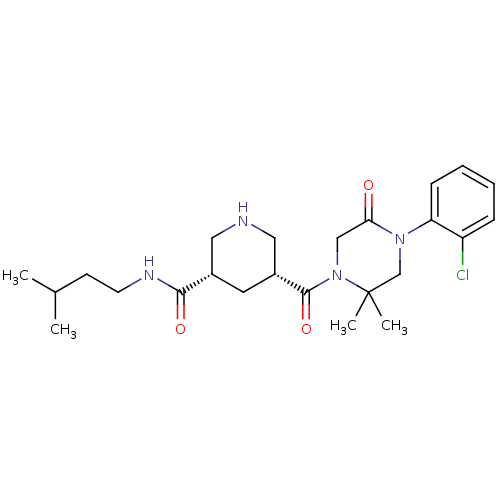 (CHEMBL2204735) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402227 (CHEMBL2204736) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 60 mins by competitive radioimmunoassay | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402228 (CHEMBL2204735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg as substrate incubated for 10 mins... | Bioorg Med Chem Lett 22: 7677-82 (2012) Article DOI: 10.1016/j.bmcl.2012.09.103 BindingDB Entry DOI: 10.7270/Q2HD7WTK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535665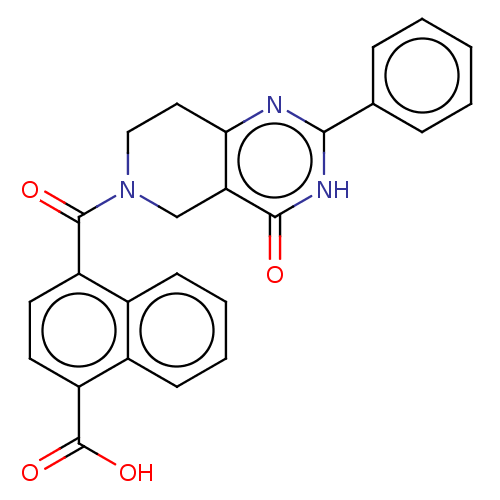 (CHEMBL4443536) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535659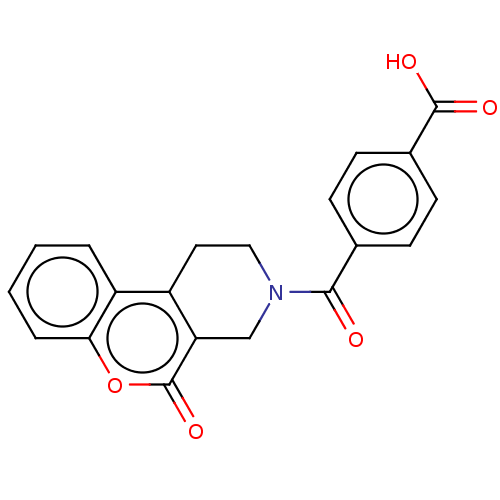 (CHEMBL4474283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535658 (CHEMBL4465667) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535670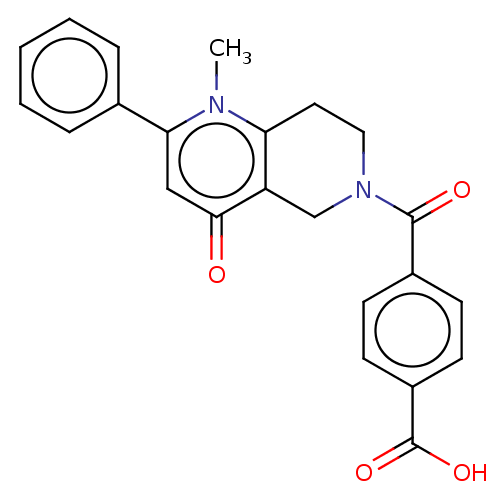 (CHEMBL4448164) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535653 (CHEMBL4530832) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535676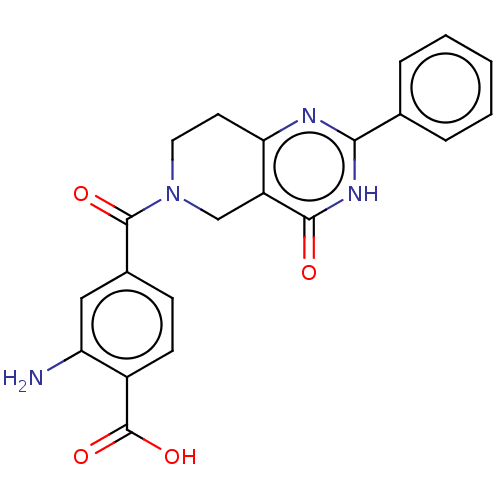 (CHEMBL4470717) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535674 (CHEMBL4538051) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535657 (CHEMBL4469262) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50535652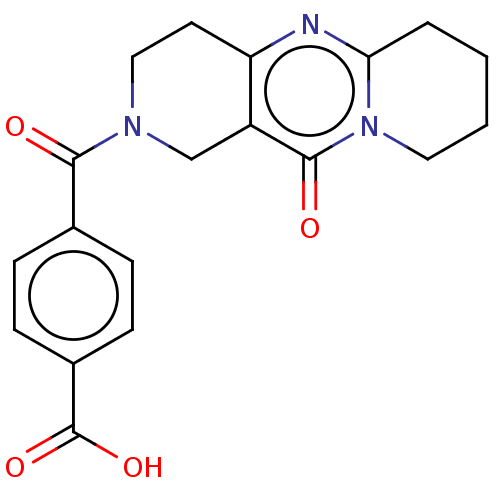 (CHEMBL4547499) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of recombinant MTHFD2 (unknown origin) assessed as reduction in methenyl-THF formation using tetrahydrofolate, NAD and formaldehyde incuba... | ACS Med Chem Lett 10: 893-898 (2019) Article DOI: 10.1021/acsmedchemlett.9b00069 BindingDB Entry DOI: 10.7270/Q2J67MFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |