 Found 169 hits with Last Name = 'mccall' and Initial = 'e'
Found 169 hits with Last Name = 'mccall' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283182
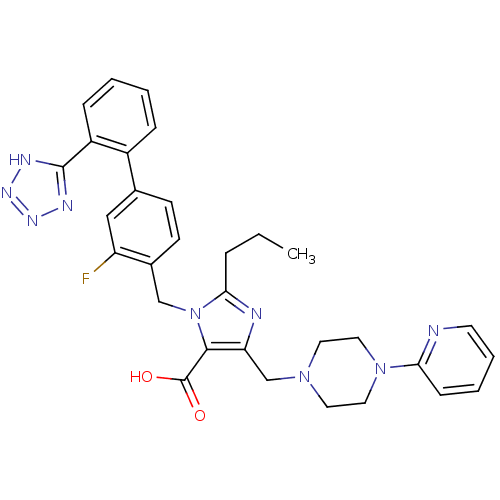
(3-[3-Fluoro-2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1F)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H32FN9O2/c1-2-7-28-34-26(20-39-14-16-40(17-15-39)27-10-5-6-13-33-27)29(31(42)43)41(28)19-22-12-11-21(18-25(22)32)23-8-3-4-9-24(23)30-35-37-38-36-30/h3-6,8-13,18H,2,7,14-17,19-20H2,1H3,(H,42,43)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283164
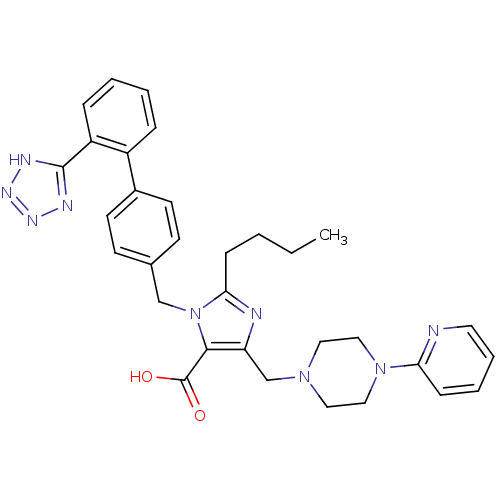
(2-Butyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-3-...)Show SMILES CCCCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H35N9O2/c1-2-3-10-29-34-27(22-39-17-19-40(20-18-39)28-11-6-7-16-33-28)30(32(42)43)41(29)21-23-12-14-24(15-13-23)25-8-4-5-9-26(25)31-35-37-38-36-31/h4-9,11-16H,2-3,10,17-22H2,1H3,(H,42,43)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283183

(2-Propyl-5-(4-pyrimidin-2-yl-piperazin-1-ylmethyl)...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ncccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H32N10O2/c1-2-6-26-33-25(20-38-15-17-39(18-16-38)30-31-13-5-14-32-30)27(29(41)42)40(26)19-21-9-11-22(12-10-21)23-7-3-4-8-24(23)28-34-36-37-35-28/h3-5,7-14H,2,6,15-20H2,1H3,(H,41,42)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283161
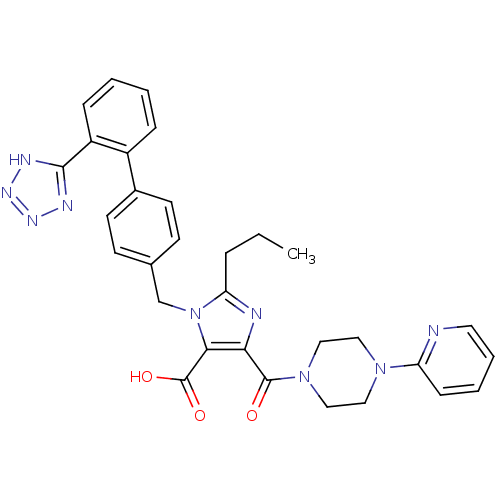
(2-Propyl-5-(4-pyridin-2-yl-piperazine-1-carbonyl)-...)Show SMILES CCCc1nc(C(=O)N2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H31N9O3/c1-2-7-26-33-27(30(41)39-18-16-38(17-19-39)25-10-5-6-15-32-25)28(31(42)43)40(26)20-21-11-13-22(14-12-21)23-8-3-4-9-24(23)29-34-36-37-35-29/h3-6,8-15H,2,7,16-20H2,1H3,(H,42,43)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283169
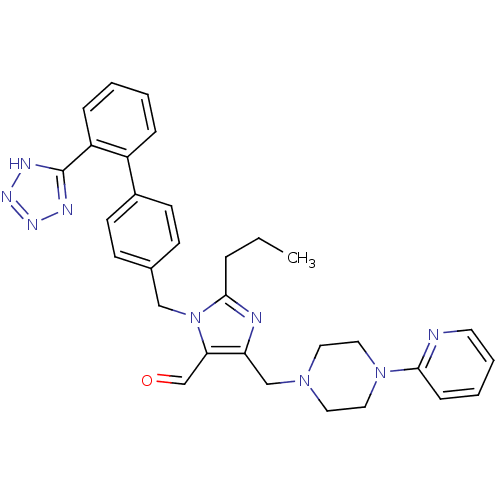
(2-Propyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-3...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(C=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H33N9O/c1-2-7-30-33-27(21-38-16-18-39(19-17-38)29-10-5-6-15-32-29)28(22-41)40(30)20-23-11-13-24(14-12-23)25-8-3-4-9-26(25)31-34-36-37-35-31/h3-6,8-15,22H,2,7,16-21H2,1H3,(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283179
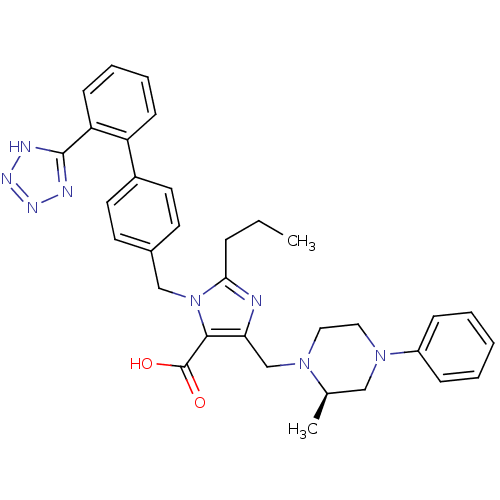
(5-((R)-2-Methyl-4-phenyl-piperazin-1-ylmethyl)-2-p...)Show SMILES CCCc1nc(CN2CCN(C[C@H]2C)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H36N8O2/c1-3-9-30-34-29(22-39-18-19-40(20-23(39)2)26-10-5-4-6-11-26)31(33(42)43)41(30)21-24-14-16-25(17-15-24)27-12-7-8-13-28(27)32-35-37-38-36-32/h4-8,10-17,23H,3,9,18-22H2,1-2H3,(H,42,43)(H,35,36,37,38)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283174
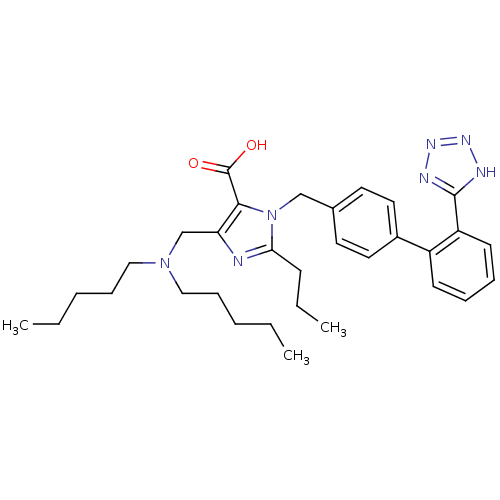
(5-Dipentylaminomethyl-2-propyl-3-[2'-(1H-tetrazol-...)Show SMILES CCCCCN(CCCCC)Cc1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1C(O)=O Show InChI InChI=1S/C32H43N7O2/c1-4-7-11-20-38(21-12-8-5-2)23-28-30(32(40)41)39(29(33-28)13-6-3)22-24-16-18-25(19-17-24)26-14-9-10-15-27(26)31-34-36-37-35-31/h9-10,14-19H,4-8,11-13,20-23H2,1-3H3,(H,40,41)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283185
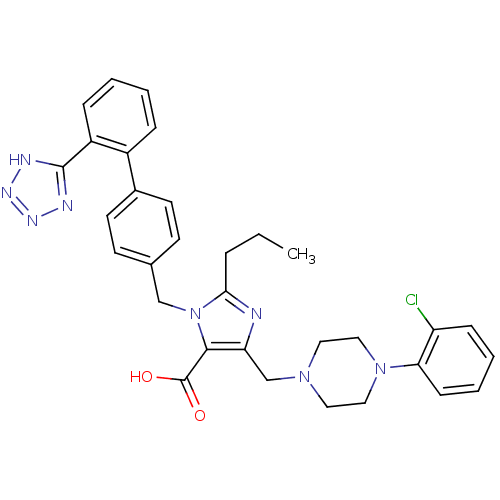
(5-[4-(2-Chloro-phenyl)-piperazin-1-ylmethyl]-2-pro...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccc2Cl)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H33ClN8O2/c1-2-7-29-34-27(21-39-16-18-40(19-17-39)28-11-6-5-10-26(28)33)30(32(42)43)41(29)20-22-12-14-23(15-13-22)24-8-3-4-9-25(24)31-35-37-38-36-31/h3-6,8-15H,2,7,16-21H2,1H3,(H,42,43)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283188
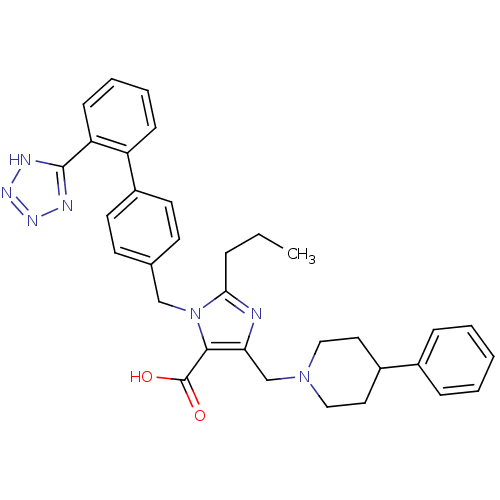
(5-(4-Phenyl-piperidin-1-ylmethyl)-2-propyl-3-[2'-(...)Show SMILES CCCc1nc(CN2CCC(CC2)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H35N7O2/c1-2-8-30-34-29(22-39-19-17-25(18-20-39)24-9-4-3-5-10-24)31(33(41)42)40(30)21-23-13-15-26(16-14-23)27-11-6-7-12-28(27)32-35-37-38-36-32/h3-7,9-16,25H,2,8,17-22H2,1H3,(H,41,42)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283162
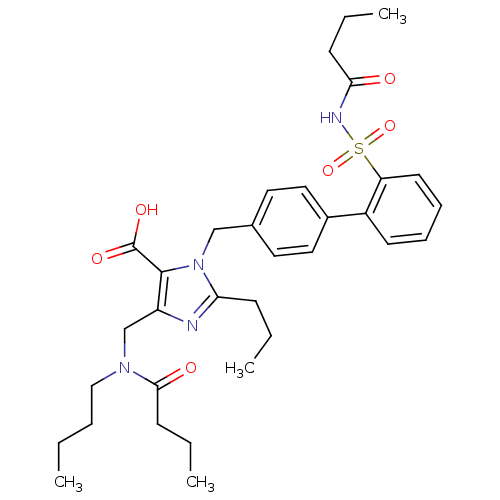
(5-[(Butyl-butyryl-amino)-methyl]-3-(2'-butyrylsulf...)Show SMILES CCCCN(Cc1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2S(=O)(=O)NC(=O)CCC)c1C(O)=O)C(=O)CCC Show InChI InChI=1S/C33H44N4O6S/c1-5-9-21-36(31(39)14-8-4)23-27-32(33(40)41)37(29(34-27)12-6-2)22-24-17-19-25(20-18-24)26-15-10-11-16-28(26)44(42,43)35-30(38)13-7-3/h10-11,15-20H,5-9,12-14,21-23H2,1-4H3,(H,35,38)(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283159
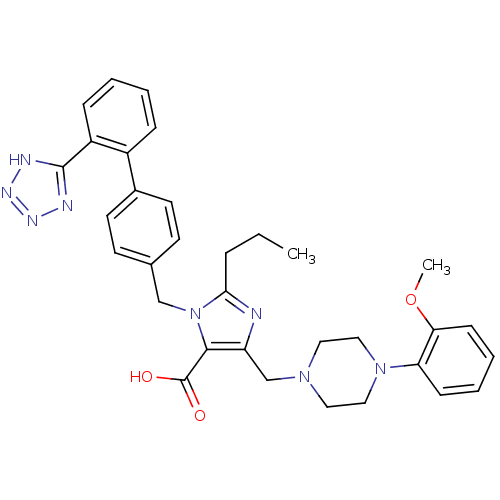
(5-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-2-pr...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccc2OC)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H36N8O3/c1-3-8-30-34-27(22-39-17-19-40(20-18-39)28-11-6-7-12-29(28)44-2)31(33(42)43)41(30)21-23-13-15-24(16-14-23)25-9-4-5-10-26(25)32-35-37-38-36-32/h4-7,9-16H,3,8,17-22H2,1-2H3,(H,42,43)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283176
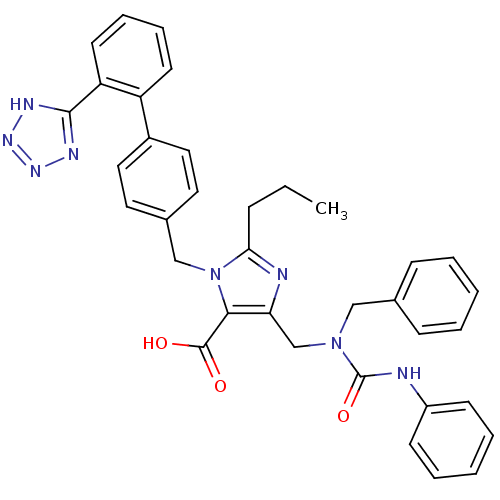
(5-(1-Benzyl-3-phenyl-ureidomethyl)-2-propyl-3-[2'-...)Show SMILES CCCc1nc(CN(Cc2ccccc2)C(=O)Nc2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H34N8O3/c1-2-11-32-38-31(24-43(22-25-12-5-3-6-13-25)36(47)37-28-14-7-4-8-15-28)33(35(45)46)44(32)23-26-18-20-27(21-19-26)29-16-9-10-17-30(29)34-39-41-42-40-34/h3-10,12-21H,2,11,22-24H2,1H3,(H,37,47)(H,45,46)(H,39,40,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283165
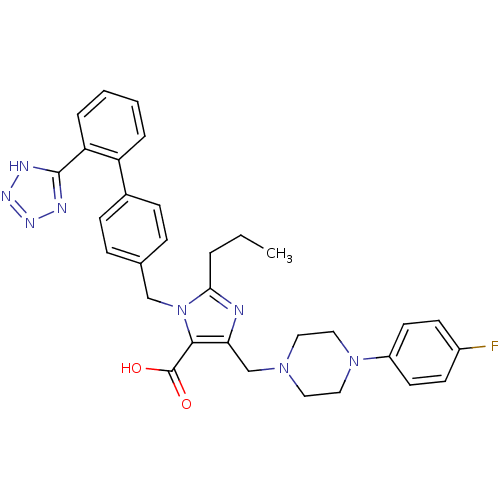
(5-[4-(4-Fluoro-phenyl)-piperazin-1-ylmethyl]-2-pro...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccc(F)cc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H33FN8O2/c1-2-5-29-34-28(21-39-16-18-40(19-17-39)25-14-12-24(33)13-15-25)30(32(42)43)41(29)20-22-8-10-23(11-9-22)26-6-3-4-7-27(26)31-35-37-38-36-31/h3-4,6-15H,2,5,16-21H2,1H3,(H,42,43)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283178
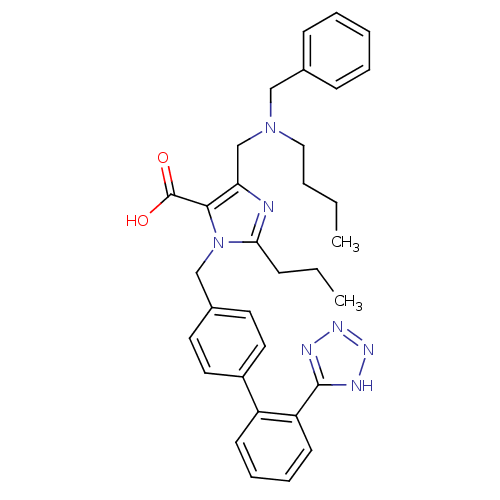
(5-[(Benzyl-butyl-amino)-methyl]-2-propyl-3-[2'-(1H...)Show SMILES CCCCN(Cc1nc(CCC)n(Cc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c1C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C33H37N7O2/c1-3-5-20-39(21-24-12-7-6-8-13-24)23-29-31(33(41)42)40(30(34-29)11-4-2)22-25-16-18-26(19-17-25)27-14-9-10-15-28(27)32-35-37-38-36-32/h6-10,12-19H,3-5,11,20-23H2,1-2H3,(H,41,42)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049178
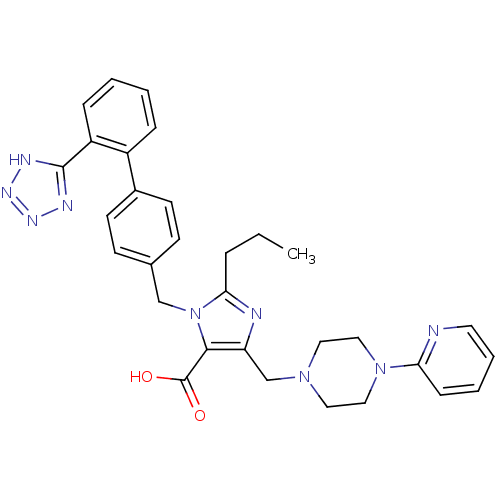
(2-Propyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-3...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H33N9O2/c1-2-7-28-33-26(21-38-16-18-39(19-17-38)27-10-5-6-15-32-27)29(31(41)42)40(28)20-22-11-13-23(14-12-22)24-8-3-4-9-25(24)30-34-36-37-35-30/h3-6,8-15H,2,7,16-21H2,1H3,(H,41,42)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283171
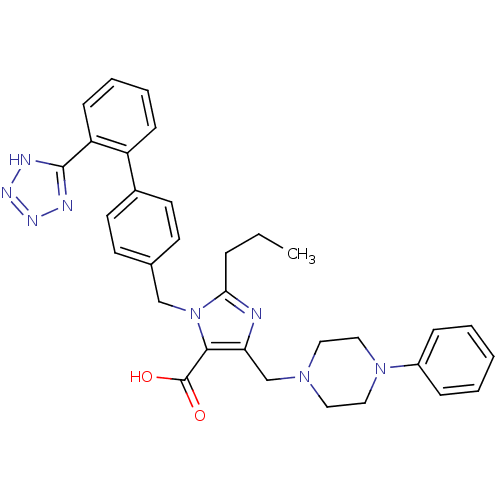
(5-(4-Phenyl-piperazin-1-ylmethyl)-2-propyl-3-[2'-(...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C32H34N8O2/c1-2-8-29-33-28(22-38-17-19-39(20-18-38)25-9-4-3-5-10-25)30(32(41)42)40(29)21-23-13-15-24(16-14-23)26-11-6-7-12-27(26)31-34-36-37-35-31/h3-7,9-16H,2,8,17-22H2,1H3,(H,41,42)(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283175
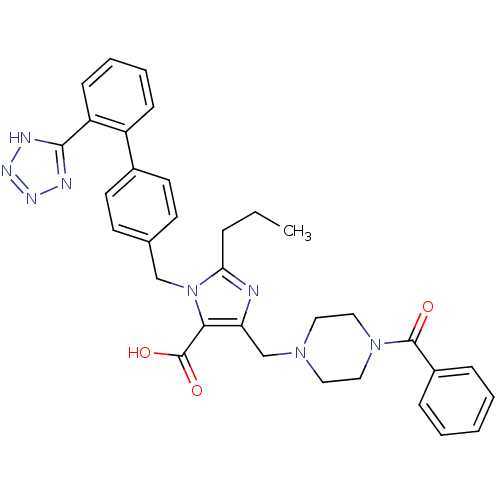
(5-(4-Benzoyl-piperazin-1-ylmethyl)-2-propyl-3-[2'-...)Show SMILES CCCc1nc(CN2CCN(CC2)C(=O)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C33H34N8O3/c1-2-8-29-34-28(22-39-17-19-40(20-18-39)32(42)25-9-4-3-5-10-25)30(33(43)44)41(29)21-23-13-15-24(16-14-23)26-11-6-7-12-27(26)31-35-37-38-36-31/h3-7,9-16H,2,8,17-22H2,1H3,(H,43,44)(H,35,36,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50406795
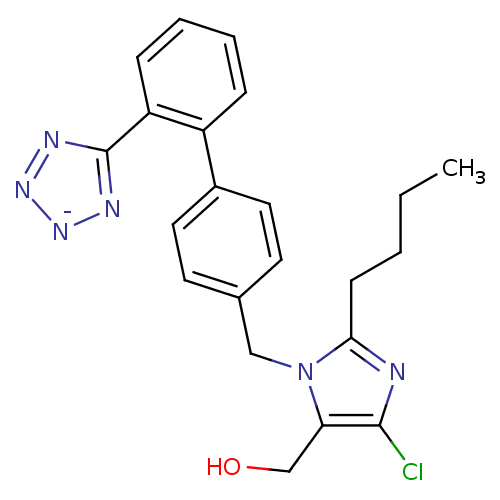
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283181
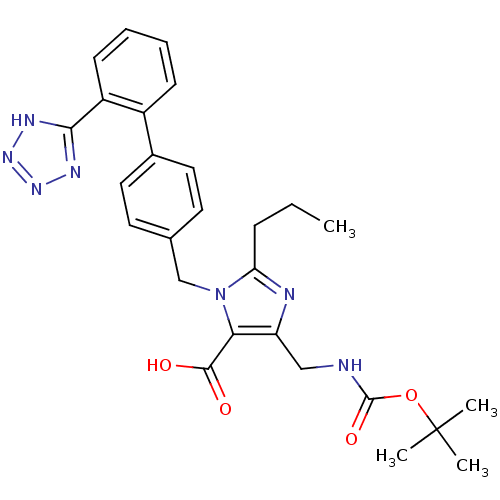
(5-(tert-Butoxycarbonylamino-methyl)-2-propyl-3-[2'...)Show SMILES CCCc1nc(CNC(=O)OC(C)(C)C)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C27H31N7O4/c1-5-8-22-29-21(15-28-26(37)38-27(2,3)4)23(25(35)36)34(22)16-17-11-13-18(14-12-17)19-9-6-7-10-20(19)24-30-32-33-31-24/h6-7,9-14H,5,8,15-16H2,1-4H3,(H,28,37)(H,35,36)(H,30,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283173
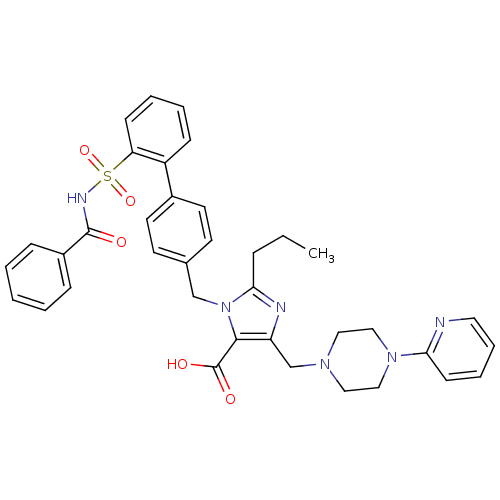
(3-(2'-Benzoylsulfamoyl-biphenyl-4-ylmethyl)-2-prop...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)c1ccccc1 Show InChI InChI=1S/C37H38N6O5S/c1-2-10-34-39-31(26-41-21-23-42(24-22-41)33-15-8-9-20-38-33)35(37(45)46)43(34)25-27-16-18-28(19-17-27)30-13-6-7-14-32(30)49(47,48)40-36(44)29-11-4-3-5-12-29/h3-9,11-20H,2,10,21-26H2,1H3,(H,40,44)(H,45,46) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283170
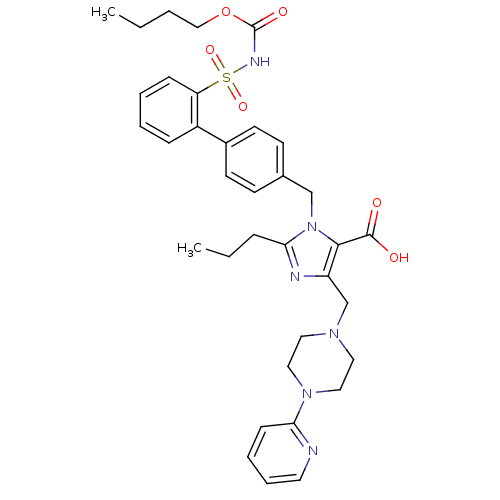
(1-[4-(2-pentanyloxycarbonylsulfamoylphenyl)benzyl]...)Show SMILES CCCCOC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CN3CCN(CC3)c3ccccn3)c2C(O)=O)cc1 Show InChI InChI=1S/C35H42N6O6S/c1-3-5-23-47-35(44)38-48(45,46)30-12-7-6-11-28(30)27-16-14-26(15-17-27)24-41-32(10-4-2)37-29(33(41)34(42)43)25-39-19-21-40(22-20-39)31-13-8-9-18-36-31/h6-9,11-18H,3-5,10,19-25H2,1-2H3,(H,38,44)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283160
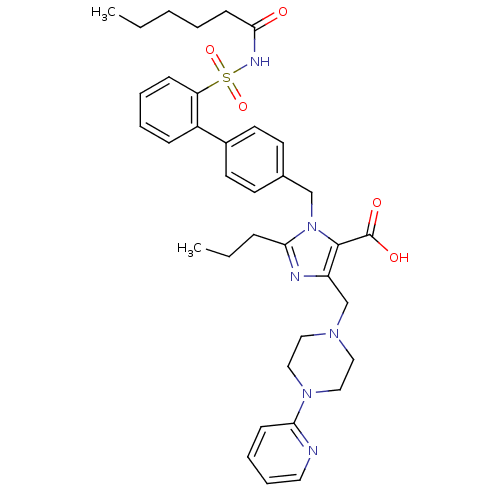
(3-(2'-Hexanoylsulfamoyl-biphenyl-4-ylmethyl)-2-pro...)Show SMILES CCCCCC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CN3CCN(CC3)c3ccccn3)c2C(O)=O)cc1 Show InChI InChI=1S/C36H44N6O5S/c1-3-5-6-15-34(43)39-48(46,47)31-13-8-7-12-29(31)28-18-16-27(17-19-28)25-42-33(11-4-2)38-30(35(42)36(44)45)26-40-21-23-41(24-22-40)32-14-9-10-20-37-32/h7-10,12-14,16-20H,3-6,11,15,21-26H2,1-2H3,(H,39,43)(H,44,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
TRAF2 and NCK-interacting protein kinase
(Homo sapiens (Human)) | BDBM50535489
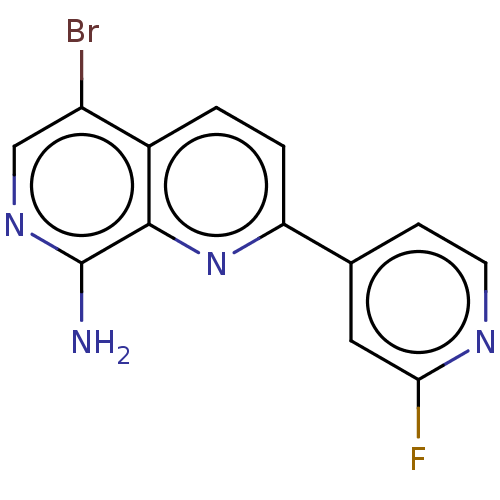
(CHEMBL4471729)Show InChI InChI=1S/C13H8BrFN4/c14-9-6-18-13(16)12-8(9)1-2-10(19-12)7-3-4-17-11(15)5-7/h1-6H,(H2,16,18) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length TNIK(1-1360)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubated... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283190
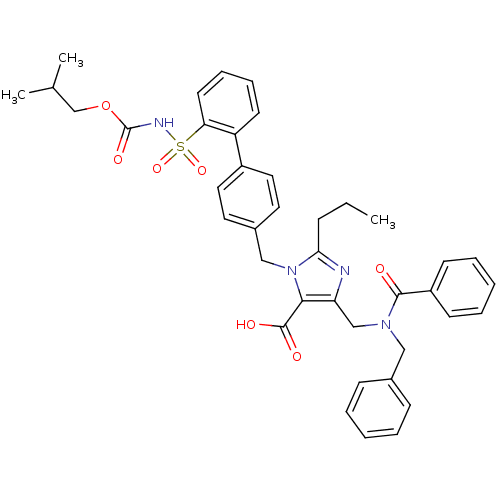
(4-benzyl(phenyl)carboxamidomethyl-1-{4-[2-(isobuty...)Show SMILES CCCc1nc(CN(Cc2ccccc2)C(=O)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)OCC(C)C Show InChI InChI=1S/C40H42N4O7S/c1-4-13-36-41-34(26-43(24-29-14-7-5-8-15-29)38(45)32-16-9-6-10-17-32)37(39(46)47)44(36)25-30-20-22-31(23-21-30)33-18-11-12-19-35(33)52(49,50)42-40(48)51-27-28(2)3/h5-12,14-23,28H,4,13,24-27H2,1-3H3,(H,42,48)(H,46,47) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283186
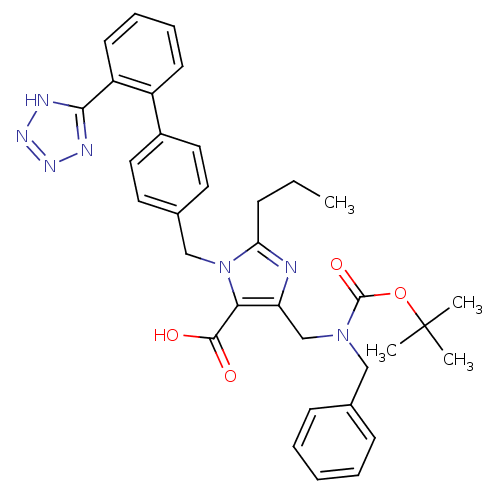
(5-[(Benzyl-tert-butoxycarbonyl-amino)-methyl]-2-pr...)Show SMILES CCCc1nc(CN(Cc2ccccc2)C(=O)OC(C)(C)C)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H37N7O4/c1-5-11-29-35-28(22-40(33(44)45-34(2,3)4)20-23-12-7-6-8-13-23)30(32(42)43)41(29)21-24-16-18-25(19-17-24)26-14-9-10-15-27(26)31-36-38-39-37-31/h6-10,12-19H,5,11,20-22H2,1-4H3,(H,42,43)(H,36,37,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
TRAF2 and NCK-interacting protein kinase
(Homo sapiens (Human)) | BDBM50138153

(CHEMBL3754283)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1N)-c1ccc(O)cc1 Show InChI InChI=1S/C18H16N2O3S/c1-24(22,23)16-8-4-13(5-9-16)17-10-14(11-20-18(17)19)12-2-6-15(21)7-3-12/h2-11,21H,1H3,(H2,19,20) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length TNIK(1-1360)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubated... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-2 angiotensin II receptor
(RAT) | BDBM50010359
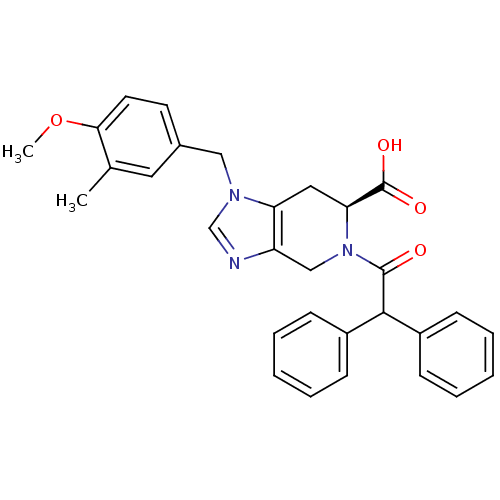
((S)-5-Diphenylacetyl-1-(4-methoxy-3-methyl-benzyl)...)Show SMILES COc1ccc(Cn2cnc3CN([C@@H](Cc23)C(O)=O)C(=O)C(c2ccccc2)c2ccccc2)cc1C Show InChI InChI=1S/C30H29N3O4/c1-20-15-21(13-14-27(20)37-2)17-32-19-31-24-18-33(26(30(35)36)16-25(24)32)29(34)28(22-9-5-3-6-10-22)23-11-7-4-8-12-23/h3-15,19,26,28H,16-18H2,1-2H3,(H,35,36)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Co.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards angitensin II AT2 receptor subtype was determined using rat isolated adrenal cortical microsomes |
J Med Chem 36: 3985-92 (1994)
BindingDB Entry DOI: 10.7270/Q2CC0ZQT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283166
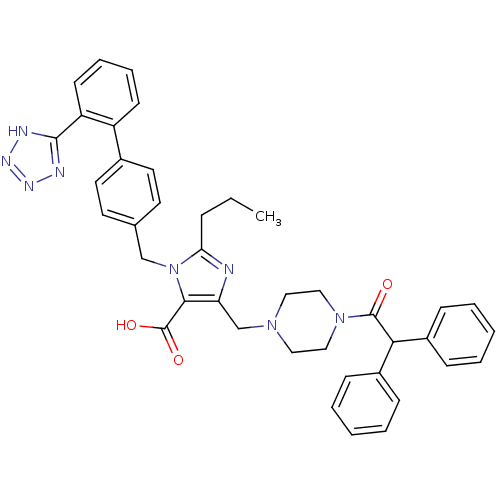
(5-(4-Diphenylacetyl-piperazin-1-ylmethyl)-2-propyl...)Show SMILES CCCc1nc(CN2CCN(CC2)C(=O)C(c2ccccc2)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C40H40N8O3/c1-2-11-35-41-34(27-46-22-24-47(25-23-46)39(49)36(30-12-5-3-6-13-30)31-14-7-4-8-15-31)37(40(50)51)48(35)26-28-18-20-29(21-19-28)32-16-9-10-17-33(32)38-42-44-45-43-38/h3-10,12-21,36H,2,11,22-27H2,1H3,(H,50,51)(H,42,43,44,45) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283168
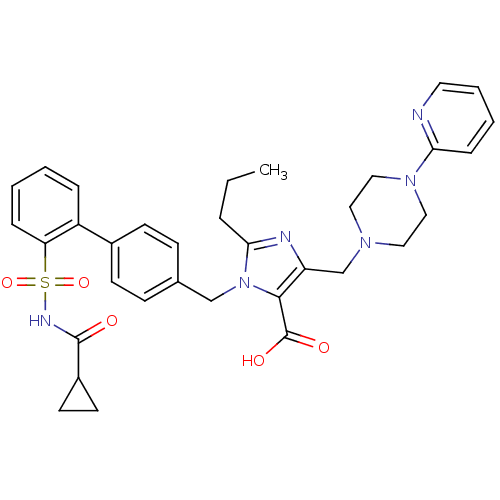
(3-[2'-(Cyclopropanecarbonyl-sulfamoyl)-biphenyl-4-...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1S(=O)(=O)NC(=O)C1CC1 Show InChI InChI=1S/C34H38N6O5S/c1-2-7-31-36-28(23-38-18-20-39(21-19-38)30-10-5-6-17-35-30)32(34(42)43)40(31)22-24-11-13-25(14-12-24)27-8-3-4-9-29(27)46(44,45)37-33(41)26-15-16-26/h3-6,8-14,17,26H,2,7,15-16,18-23H2,1H3,(H,37,41)(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283184

(CHEMBL72409 | {2-Propyl-5-(4-pyridin-2-yl-piperazi...)Show SMILES CCCc1nc(CN2CCN(CC2)c2ccccn2)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C31H35N9O/c1-2-7-30-33-27(21-38-16-18-39(19-17-38)29-10-5-6-15-32-29)28(22-41)40(30)20-23-11-13-24(14-12-23)25-8-3-4-9-26(25)31-34-36-37-35-31/h3-6,8-15,41H,2,7,16-22H2,1H3,(H,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283180
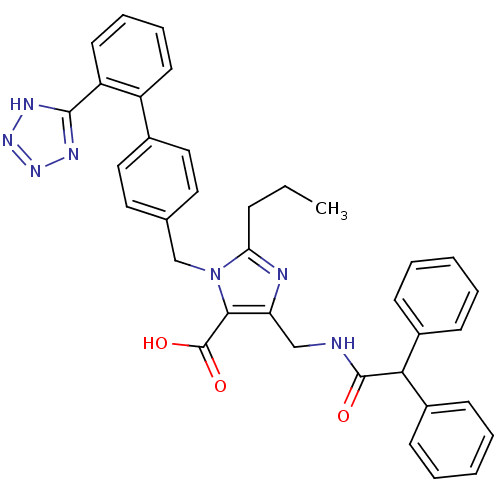
(5-(Diphenylacetylamino-methyl)-2-propyl-3-[2'-(1H-...)Show SMILES CCCc1nc(CNC(=O)C(c2ccccc2)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C36H33N7O3/c1-2-11-31-38-30(22-37-35(44)32(26-12-5-3-6-13-26)27-14-7-4-8-15-27)33(36(45)46)43(31)23-24-18-20-25(21-19-24)28-16-9-10-17-29(28)34-39-41-42-40-34/h3-10,12-21,32H,2,11,22-23H2,1H3,(H,37,44)(H,45,46)(H,39,40,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283187
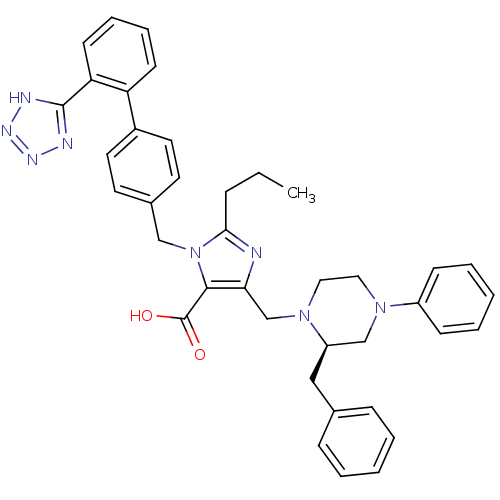
(5-((R)-2-Benzyl-4-phenyl-piperazin-1-ylmethyl)-2-p...)Show SMILES CCCc1nc(CN2CCN(C[C@H]2Cc2ccccc2)c2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C39H40N8O2/c1-2-11-36-40-35(27-46-23-22-45(31-14-7-4-8-15-31)26-32(46)24-28-12-5-3-6-13-28)37(39(48)49)47(36)25-29-18-20-30(21-19-29)33-16-9-10-17-34(33)38-41-43-44-42-38/h3-10,12-21,32H,2,11,22-27H2,1H3,(H,48,49)(H,41,42,43,44)/t32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283172
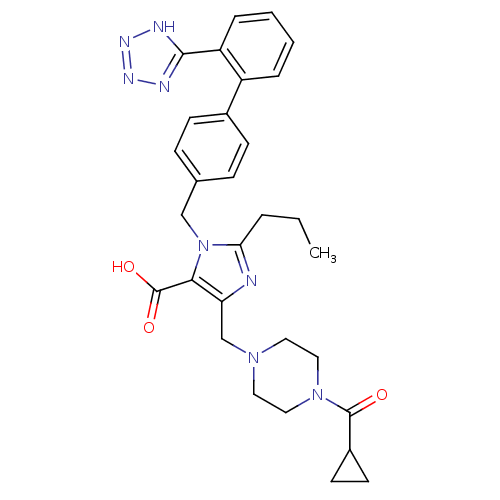
(5-(4-Cyclopropanecarbonyl-piperazin-1-ylmethyl)-2-...)Show SMILES CCCc1nc(CN2CCN(CC2)C(=O)C2CC2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H34N8O3/c1-2-5-26-31-25(19-36-14-16-37(17-15-36)29(39)22-12-13-22)27(30(40)41)38(26)18-20-8-10-21(11-9-20)23-6-3-4-7-24(23)28-32-34-35-33-28/h3-4,6-11,22H,2,5,12-19H2,1H3,(H,40,41)(H,32,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
TRAF2 and NCK-interacting protein kinase
(Homo sapiens (Human)) | BDBM50138153

(CHEMBL3754283)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1N)-c1ccc(O)cc1 Show InChI InChI=1S/C18H16N2O3S/c1-24(22,23)16-8-4-13(5-9-16)17-10-14(11-20-18(17)19)12-2-6-15(21)7-3-12/h2-11,21H,1H3,(H2,19,20) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-TEV-TNIK(1 to 325) expressed in baculovirus-infected sf21 cells using LRRKtide as substrate preincubated for 30 mins followe... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50535489

(CHEMBL4471729)Show InChI InChI=1S/C13H8BrFN4/c14-9-6-18-13(16)12-8(9)1-2-10(19-12)7-3-4-17-11(15)5-7/h1-6H,(H2,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MINK1(1-1332)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubate... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50535491
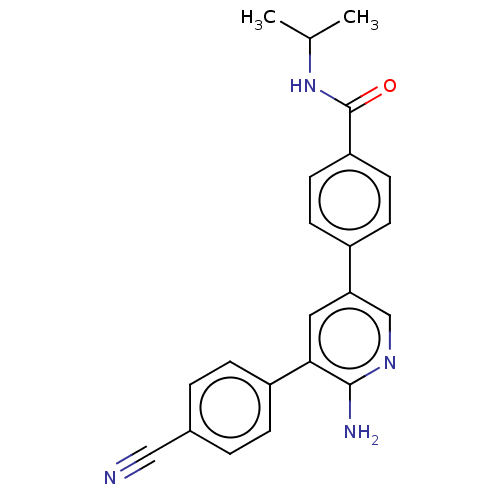
(CHEMBL4558962)Show SMILES CC(C)NC(=O)c1ccc(cc1)-c1cnc(N)c(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C22H20N4O/c1-14(2)26-22(27)18-9-7-16(8-10-18)19-11-20(21(24)25-13-19)17-5-3-15(12-23)4-6-17/h3-11,13-14H,1-2H3,(H2,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-TEV-SHM-MAP4K4(2 to 328)-GNS expressed in baculovirus-infected sf21 cells using LRRKtide as substrate preincubated for 30 mi... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | |
TRAF2 and NCK-interacting protein kinase
(Homo sapiens (Human)) | BDBM50535489

(CHEMBL4471729)Show InChI InChI=1S/C13H8BrFN4/c14-9-6-18-13(16)12-8(9)1-2-10(19-12)7-3-4-17-11(15)5-7/h1-6H,(H2,16,18) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-TEV-TNIK(1 to 325) expressed in baculovirus-infected sf21 cells using LRRKtide as substrate preincubated for 30 mins followe... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50138153

(CHEMBL3754283)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1N)-c1ccc(O)cc1 Show InChI InChI=1S/C18H16N2O3S/c1-24(22,23)16-8-4-13(5-9-16)17-10-14(11-20-18(17)19)12-2-6-15(21)7-3-12/h2-11,21H,1H3,(H2,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MINK1(1-1332)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubate... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50138153

(CHEMBL3754283)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1N)-c1ccc(O)cc1 Show InChI InChI=1S/C18H16N2O3S/c1-24(22,23)16-8-4-13(5-9-16)17-10-14(11-20-18(17)19)12-2-6-15(21)7-3-12/h2-11,21H,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MAP4K4(1-1239)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubat... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50535489

(CHEMBL4471729)Show InChI InChI=1S/C13H8BrFN4/c14-9-6-18-13(16)12-8(9)1-2-10(19-12)7-3-4-17-11(15)5-7/h1-6H,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MAP4K4(1-1239)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubat... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283189
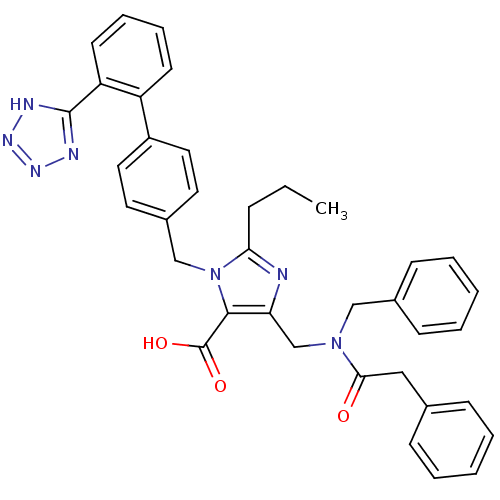
(5-[(Benzyl-phenylacetyl-amino)-methyl]-2-propyl-3-...)Show SMILES CCCc1nc(CN(Cc2ccccc2)C(=O)Cc2ccccc2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C37H35N7O3/c1-2-11-33-38-32(25-43(23-27-14-7-4-8-15-27)34(45)22-26-12-5-3-6-13-26)35(37(46)47)44(33)24-28-18-20-29(21-19-28)30-16-9-10-17-31(30)36-39-41-42-40-36/h3-10,12-21H,2,11,22-25H2,1H3,(H,46,47)(H,39,40,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283167
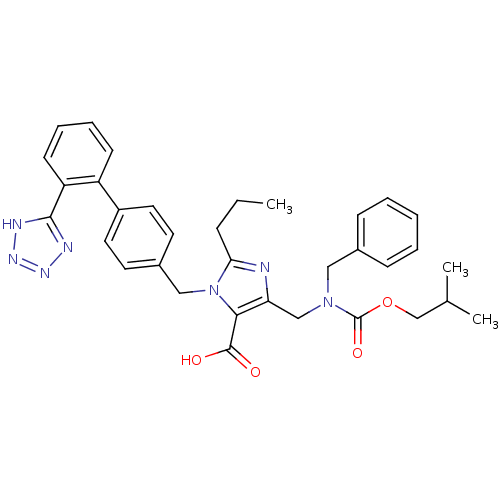
(5-[(Benzyl-isobutoxycarbonyl-amino)-methyl]-2-prop...)Show SMILES CCCc1nc(CN(Cc2ccccc2)C(=O)OCC(C)C)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C34H37N7O4/c1-4-10-30-35-29(21-40(34(44)45-22-23(2)3)19-24-11-6-5-7-12-24)31(33(42)43)41(30)20-25-15-17-26(18-16-25)27-13-8-9-14-28(27)32-36-38-39-37-32/h5-9,11-18,23H,4,10,19-22H2,1-3H3,(H,42,43)(H,36,37,38,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283163
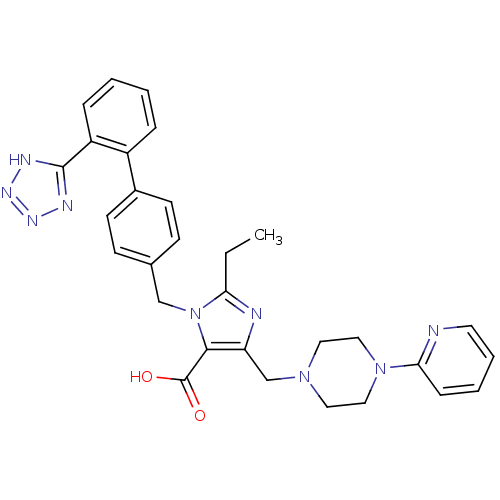
(2-Ethyl-5-(4-pyridin-2-yl-piperazin-1-ylmethyl)-3-...)Show SMILES CCc1nc(CN2CCN(CC2)c2ccccn2)c(C(O)=O)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C30H31N9O2/c1-2-26-32-25(20-37-15-17-38(18-16-37)27-9-5-6-14-31-27)28(30(40)41)39(26)19-21-10-12-22(13-11-21)23-7-3-4-8-24(23)29-33-35-36-34-29/h3-14H,2,15-20H2,1H3,(H,40,41)(H,33,34,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against Angiotensin II receptor, type 1 |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50283160

(3-(2'-Hexanoylsulfamoyl-biphenyl-4-ylmethyl)-2-pro...)Show SMILES CCCCCC(=O)NS(=O)(=O)c1ccccc1-c1ccc(Cn2c(CCC)nc(CN3CCN(CC3)c3ccccn3)c2C(O)=O)cc1 Show InChI InChI=1S/C36H44N6O5S/c1-3-5-6-15-34(43)39-48(46,47)31-13-8-7-12-29(31)28-18-16-27(17-19-28)25-42-33(11-4-2)38-30(35(42)36(44)45)26-40-21-23-41(24-22-40)32-14-9-10-20-37-32/h7-10,12-14,16-20H,3-6,11,15,21-26H2,1-2H3,(H,39,43)(H,44,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity of compound against angiotensin II AT-2 receptor |
Bioorg Med Chem Lett 4: 2229-2234 (1994)
Article DOI: 10.1016/S0960-894X(00)80076-1
BindingDB Entry DOI: 10.7270/Q2JQ110M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50535491

(CHEMBL4558962)Show SMILES CC(C)NC(=O)c1ccc(cc1)-c1cnc(N)c(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C22H20N4O/c1-14(2)26-22(27)18-9-7-16(8-10-18)19-11-20(21(24)25-13-19)17-5-3-15(12-23)4-6-17/h3-11,13-14H,1-2H3,(H2,24,25)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MAP4K4(1-1239)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubat... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50043046
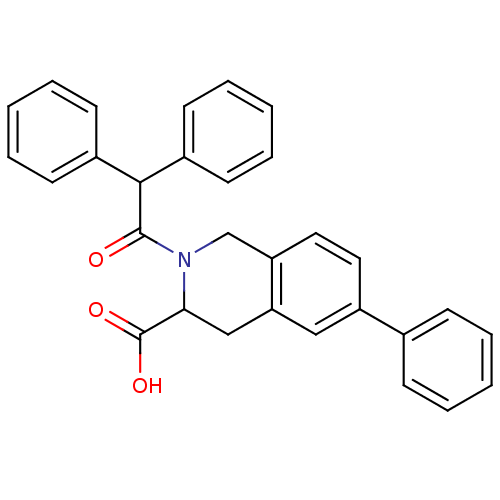
(2-Diphenylacetyl-6-phenyl-1,2,3,4-tetrahydro-isoqu...)Show SMILES OC(=O)C1Cc2cc(ccc2CN1C(=O)C(c1ccccc1)c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C30H25NO3/c32-29(28(22-12-6-2-7-13-22)23-14-8-3-9-15-23)31-20-25-17-16-24(21-10-4-1-5-11-21)18-26(25)19-27(31)30(33)34/h1-18,27-28H,19-20H2,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Co.
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II receptor, type 2 in rat isolated adrenal medullary microsomes |
J Med Chem 36: 3985-92 (1994)
BindingDB Entry DOI: 10.7270/Q2CC0ZQT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50138153

(CHEMBL3754283)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(cnc1N)-c1ccc(O)cc1 Show InChI InChI=1S/C18H16N2O3S/c1-24(22,23)16-8-4-13(5-9-16)17-10-14(11-20-18(17)19)12-2-6-15(21)7-3-12/h2-11,21H,1H3,(H2,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-TEV-SHM-MAP4K4(2 to 328)-GNS expressed in baculovirus-infected sf21 cells using LRRKtide as substrate preincubated for 30 mi... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase kinase kinase kinase 4
(Homo sapiens (Human)) | BDBM50535489

(CHEMBL4471729)Show InChI InChI=1S/C13H8BrFN4/c14-9-6-18-13(16)12-8(9)1-2-10(19-12)7-3-4-17-11(15)5-7/h1-6H,(H2,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 6His-TEV-SHM-MAP4K4(2 to 328)-GNS expressed in baculovirus-infected sf21 cells using LRRKtide as substrate preincubated for 30 mi... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Misshapen-like kinase 1
(Homo sapiens (Human)) | BDBM50535491

(CHEMBL4558962)Show SMILES CC(C)NC(=O)c1ccc(cc1)-c1cnc(N)c(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C22H20N4O/c1-14(2)26-22(27)18-9-7-16(8-10-18)19-11-20(21(24)25-13-19)17-5-3-15(12-23)4-6-17/h3-11,13-14H,1-2H3,(H2,24,25)(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human full length MINK1(1-1332)-G5-Avi-6His-Flag expressed in baculovirus-infected sf21 cells using His6-SMAD1 as substrate preincubate... |
Bioorg Med Chem Lett 29: 1962-1967 (2019)
Article DOI: 10.1016/j.bmcl.2019.05.032
BindingDB Entry DOI: 10.7270/Q2PZ5DBT |
More data for this
Ligand-Target Pair | |
Type-2 angiotensin II receptor
(RAT) | BDBM50043034
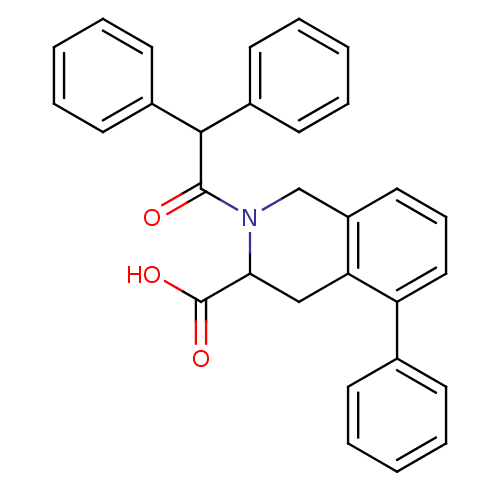
(2-Diphenylacetyl-5-phenyl-1,2,3,4-tetrahydro-isoqu...)Show SMILES OC(=O)C1Cc2c(CN1C(=O)C(c1ccccc1)c1ccccc1)cccc2-c1ccccc1 Show InChI InChI=1S/C30H25NO3/c32-29(28(22-13-6-2-7-14-22)23-15-8-3-9-16-23)31-20-24-17-10-18-25(21-11-4-1-5-12-21)26(24)19-27(31)30(33)34/h1-18,27-28H,19-20H2,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Co.
Curated by ChEMBL
| Assay Description
Binding affinity towards Angiotensin II receptor, type 2 in rat isolated adrenal medullary microsomes |
J Med Chem 36: 3985-92 (1994)
BindingDB Entry DOI: 10.7270/Q2CC0ZQT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data