

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from rat adenosine A1 receptor | Eur J Med Chem 43: 614-20 (2008) Article DOI: 10.1016/j.ejmech.2007.05.001 BindingDB Entry DOI: 10.7270/Q22Z15BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]MSX2 from rat adenosine A2A receptor | Eur J Med Chem 43: 614-20 (2008) Article DOI: 10.1016/j.ejmech.2007.05.001 BindingDB Entry DOI: 10.7270/Q22Z15BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50260483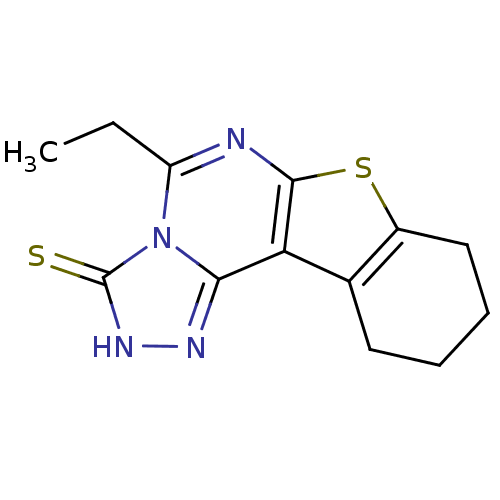 (5-Ethyl-8,9,10,11-tetrahydro[1]benzothieno[3,2-e][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from rat adenosine A1 receptor | Eur J Med Chem 43: 614-20 (2008) Article DOI: 10.1016/j.ejmech.2007.05.001 BindingDB Entry DOI: 10.7270/Q22Z15BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50260482 (5-Ethyl-8,9-dimethylthieno[3,2-e][1,2,4]triazolo[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from rat adenosine A1 receptor | Eur J Med Chem 43: 614-20 (2008) Article DOI: 10.1016/j.ejmech.2007.05.001 BindingDB Entry DOI: 10.7270/Q22Z15BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50260481 (5-Methyl-8,9,10,11-tetrahydro[1]benzothieno[3,2-e]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from rat adenosine A1 receptor | Eur J Med Chem 43: 614-20 (2008) Article DOI: 10.1016/j.ejmech.2007.05.001 BindingDB Entry DOI: 10.7270/Q22Z15BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50608608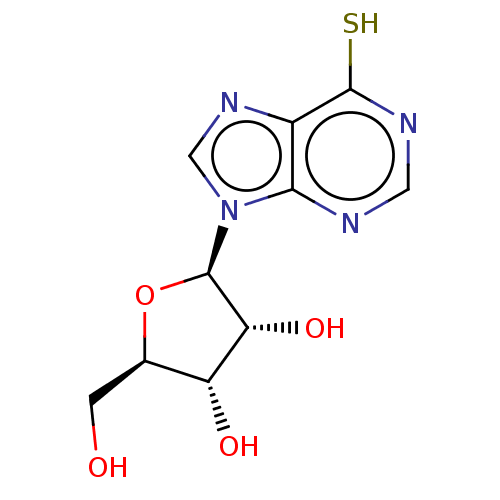 (THIOINOSINE | Thioinosine) | UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50423778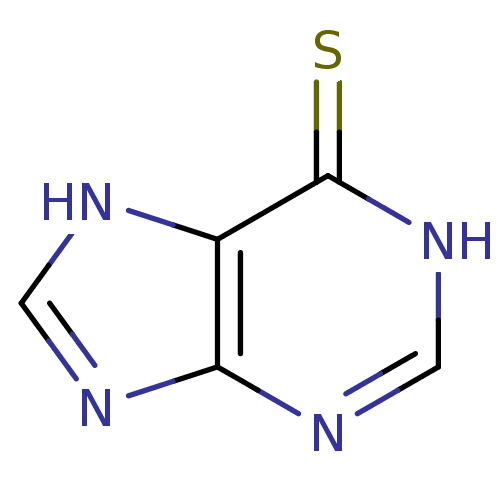 (Leukerin | MERCAPTOPURINE | Mercaleukin | Mercapto...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid UniChem | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50200099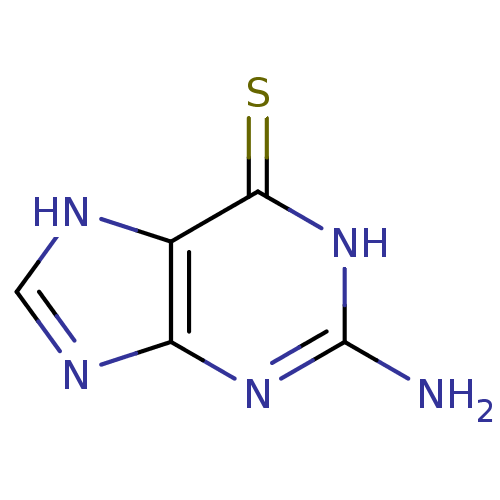 (2-Amino-1,7-dihydro-purine-6-thione | 2-Amino-1,9-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50373919 (AZATHIOPRINE | Azasan) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM384362 (US9932327, Compound 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-9/Cyclin T1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA, 0.1% mercaptoethanol) was assayed against a substrate peptide (YSPTS... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM384375 (US9932327, Compound 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-9/Cyclin T1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA, 0.1% mercaptoethanol) was assayed against a substrate peptide (YSPTS... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM384362 (US9932327, Compound 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-2/cyclin A (5-20 mU diluted in 50 mM Hepes pH 7.5, 1 mM DTT, 0.02% Brij35, 100 mM NaCl) was assayed against Histone H1 in a final volume of 25.5 ... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM384357 (US9932327, Compound Flavopiridol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-9/Cyclin T1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA, 0.1% mercaptoethanol) was assayed against a substrate peptide (YSPTS... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of COX2 (unknown origin) | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM384376 (US9932327, Compound 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-9/Cyclin T1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA, 0.1% mercaptoethanol) was assayed against a substrate peptide (YSPTS... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM384357 (US9932327, Compound Flavopiridol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-2/cyclin A (5-20 mU diluted in 50 mM Hepes pH 7.5, 1 mM DTT, 0.02% Brij35, 100 mM NaCl) was assayed against Histone H1 in a final volume of 25.5 ... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome c oxidase subunit 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 2 (Homo sapiens (Human)) | BDBM50515743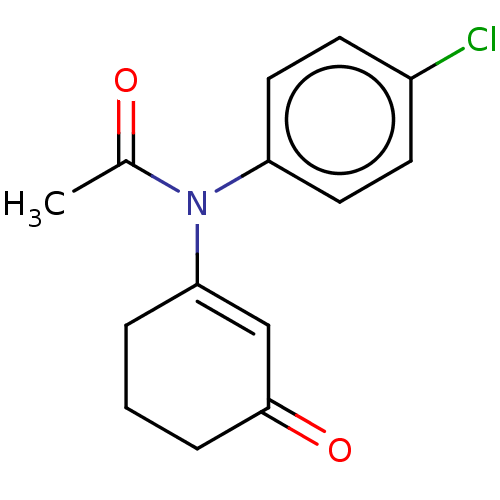 (CHEMBL4448990) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 367 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019936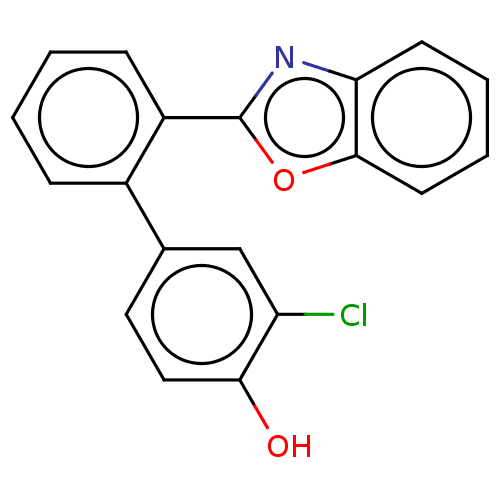 (CHEMBL3287559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM384376 (US9932327, Compound 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 466 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-2/cyclin A (5-20 mU diluted in 50 mM Hepes pH 7.5, 1 mM DTT, 0.02% Brij35, 100 mM NaCl) was assayed against Histone H1 in a final volume of 25.5 ... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 2 (Homo sapiens (Human)) | BDBM50515744 (CHEMBL4441570) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM384315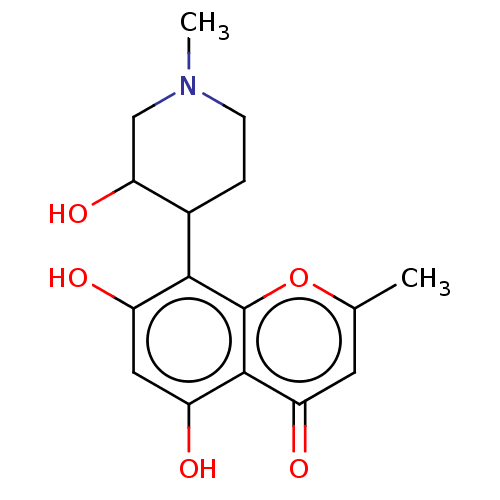 (US9932327, Compound Rohitukine (1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-9/Cyclin T1 (5-20 mU diluted in 50 mM Tris pH 7.5, 0.1 mM EGTA, 1 mg/ml BSA, 0.1% mercaptoethanol) was assayed against a substrate peptide (YSPTS... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM384375 (US9932327, Compound 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-2/cyclin A (5-20 mU diluted in 50 mM Hepes pH 7.5, 1 mM DTT, 0.02% Brij35, 100 mM NaCl) was assayed against Histone H1 in a final volume of 25.5 ... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019935 (CHEMBL3287567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 2 (Homo sapiens (Human)) | BDBM50515745 (CHEMBL4474644) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 915 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of recombinant human COX2 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50019937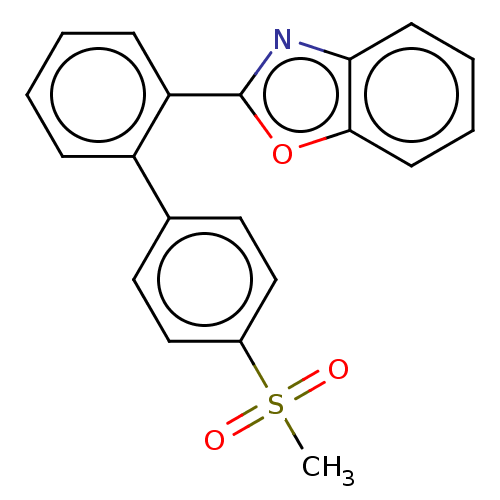 (CHEMBL3287560) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM384315 (US9932327, Compound Rohitukine (1)) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze | Assay Description CDK-2/cyclin A (5-20 mU diluted in 50 mM Hepes pH 7.5, 1 mM DTT, 0.02% Brij35, 100 mM NaCl) was assayed against Histone H1 in a final volume of 25.5 ... | Bioorg Med Chem Lett 18: 2567-73 (2008) BindingDB Entry DOI: 10.7270/Q25Q4ZDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50247903 (Amithiozone | Thiacetazone | Thioacetazone) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50608606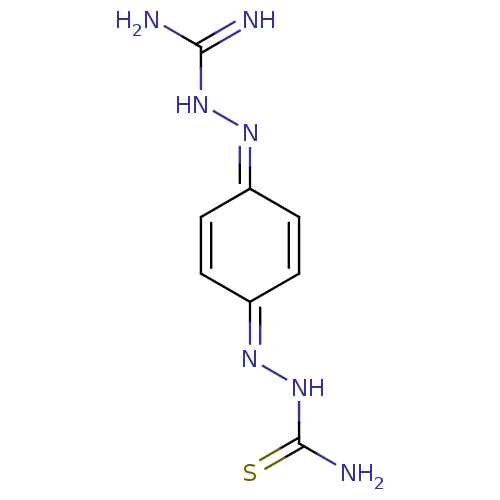 (AMBAZONE | Ambazone | Faringosept) | UniProtKB/SwissProt GoogleScholar AffyNet | UniChem | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of COX1 (unknown origin) | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Ovis aries) | BDBM50515745 (CHEMBL4474644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50523087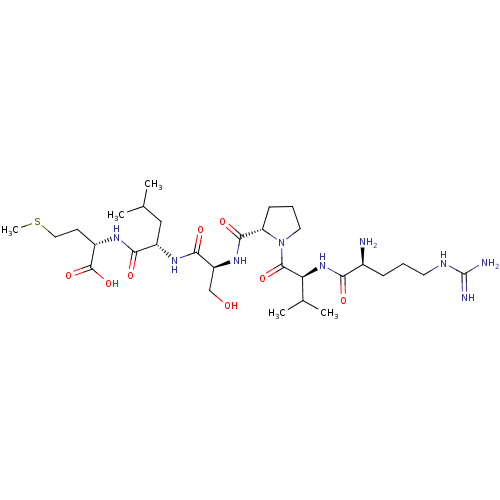 (CHEMBL4458477) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goa University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) incubated for 20 mins before pNPG substrate addition and measured after 30 mins by UV spectrometry | Bioorg Med Chem 27: 2340-2344 (2019) Article DOI: 10.1016/j.bmc.2018.12.021 BindingDB Entry DOI: 10.7270/Q2Q243N3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Ovis aries) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Ovis aries) | BDBM50515744 (CHEMBL4441570) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome c oxidase subunit 1 (Ovis aries) | BDBM50515743 (CHEMBL4448990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 assessed as reduction in PGF2alpha incubated for 2 mins using arachidonic acid as substrate by ELISA | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111601 BindingDB Entry DOI: 10.7270/Q20V8H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019937 (CHEMBL3287560) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019936 (CHEMBL3287559) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50019935 (CHEMBL3287567) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER) Curated by ChEMBL | Assay Description Inhibition of ovine COX1 using arachidonic acid as substrate | ACS Med Chem Lett 5: 512-6 (2014) Article DOI: 10.1021/ml400500e BindingDB Entry DOI: 10.7270/Q23R0VF9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Probable maltase-glucoamylase 2 (Homo sapiens) | BDBM50022184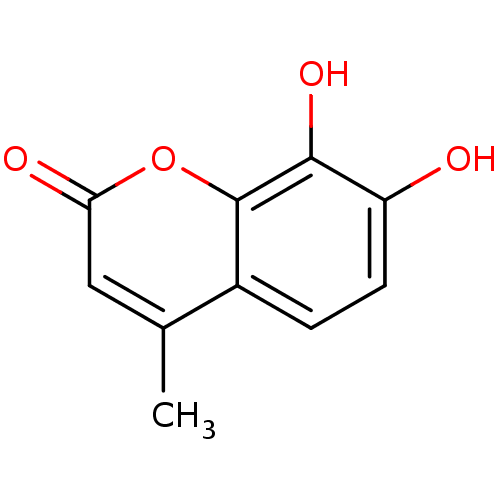 (7,8-Dihydroxy-4-methyl-2H-chromen-2-one (3) | 7,8-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Goa University Curated by ChEMBL | Assay Description Inhibition of alpha-glucosidase (unknown origin) incubated for 20 mins before pNPG substrate addition and measured after 30 mins by UV spectrometry | Bioorg Med Chem 27: 2340-2344 (2019) Article DOI: 10.1016/j.bmc.2018.12.021 BindingDB Entry DOI: 10.7270/Q2Q243N3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50241361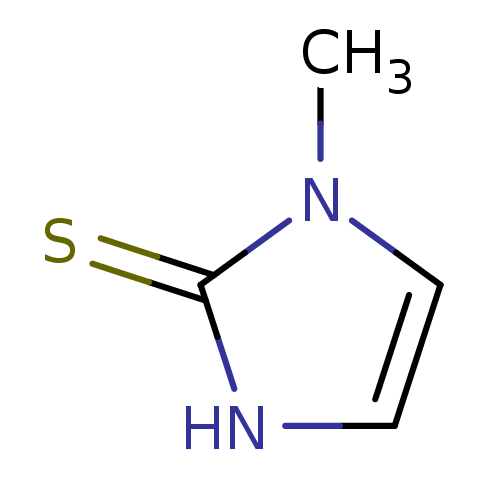 (CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50608607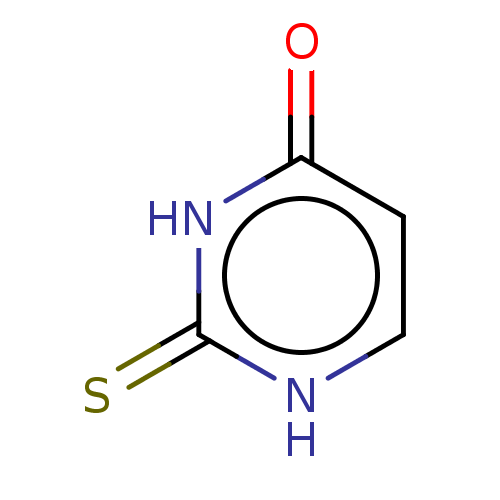 (CHEBI:348530 | THIOURACIL | Thiouracil) | UniProtKB/SwissProt GoogleScholar AffyNet | PDB UniChem | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50239994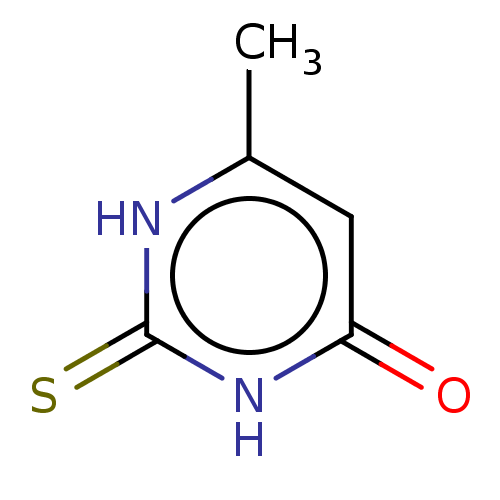 (CHEBI:82346 | Methylthiouracil) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | n/a | n/a | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50133597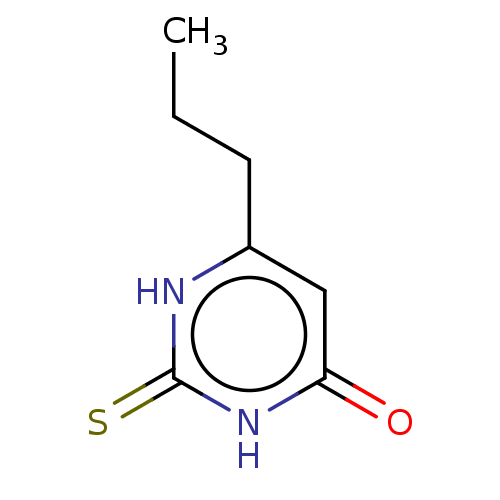 (CHEBI:8502 | Propacil | Propylthiouracil | Prothyr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50275889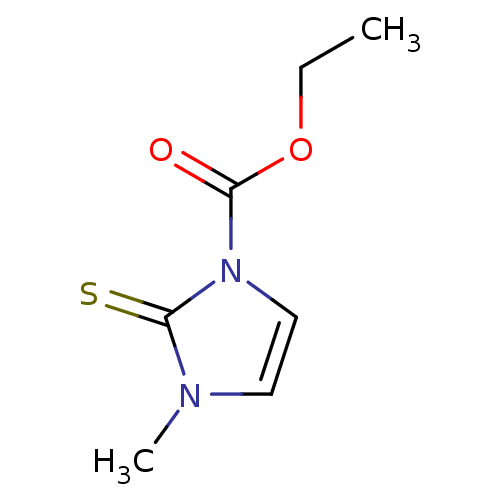 (CHEMBL508102 | carbimazole) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | n/a | n/a | 1.86E+5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||