

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50086170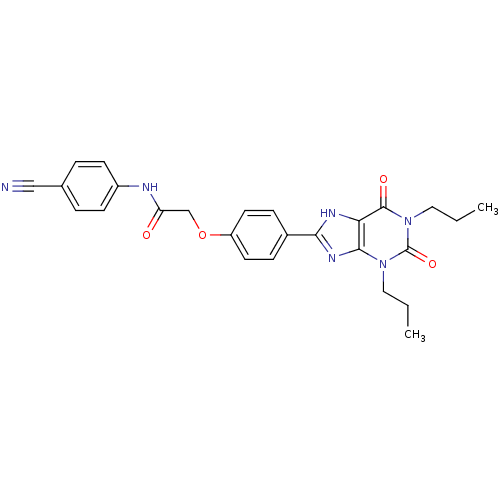 ((4-Cyano-phenyl)-carbamic acid 4-(2,6-dioxo-1,3-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM520995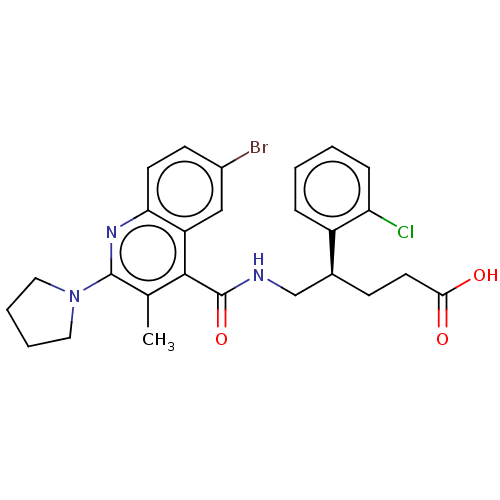 ((+)-5-[({6-Bromo-3-methyl-2-[(2H8)pyrrolidin-1-yl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]PGF2alpha from full-length recombinant human FP receptor expressed in HEK293 cell membranes measured after 60 mins by scintillati... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00834 BindingDB Entry DOI: 10.7270/Q2FJ2MC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50336977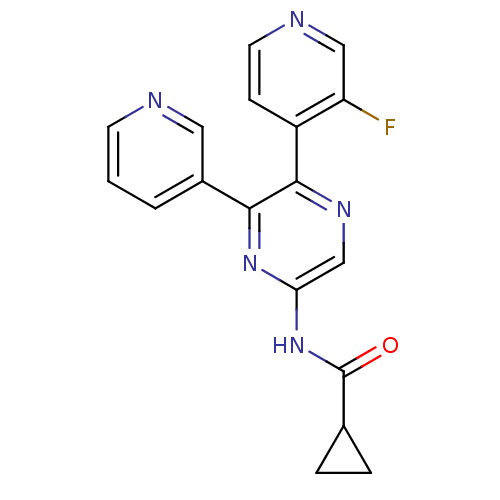 (CHEMBL1672627 | N-[5-(3-Fluoropyridin-4-yl)-6-pyri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50531422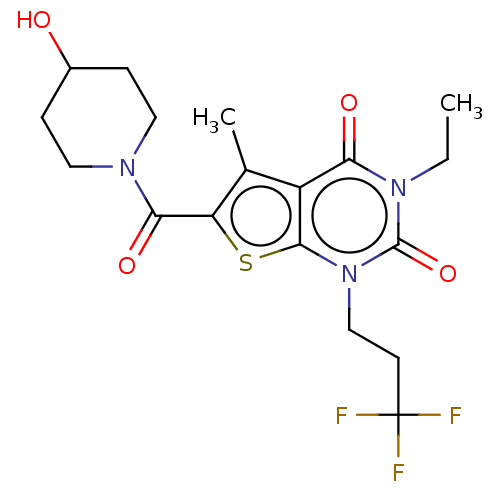 (CHEMBL4522981) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CGS21680 from human A2A adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human A1 adenosine receptor expressed in CHO cell membranes after 60 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50531422 (CHEMBL4522981) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human A3 adenosine receptor expressed in HEK293 cell membranes after 120 mins by radioligand displacement assay | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM10849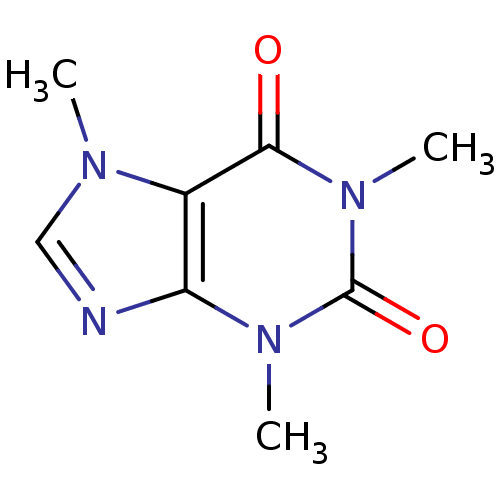 (1,3,7-trimethyl-2,3,6,7-tetrahydro-1H-purine-2,6-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human A2B adenosine receptor | Eur J Med Chem 163: 763-778 (2019) Article DOI: 10.1016/j.ejmech.2018.11.045 BindingDB Entry DOI: 10.7270/Q24J0JKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413625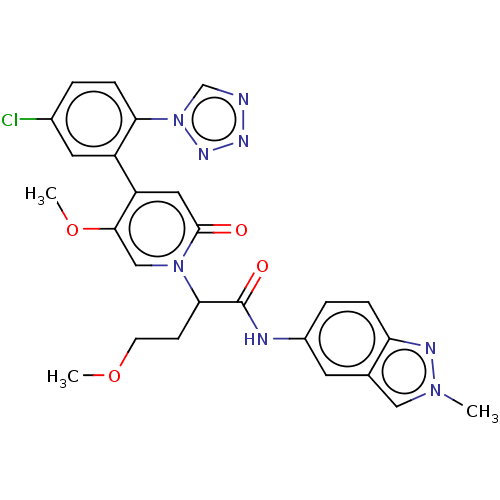 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413725 (N-(Quinoxalin-6-yl)-(2S)-2-{4-[5-chloro-2-(1H-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413625 (2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189921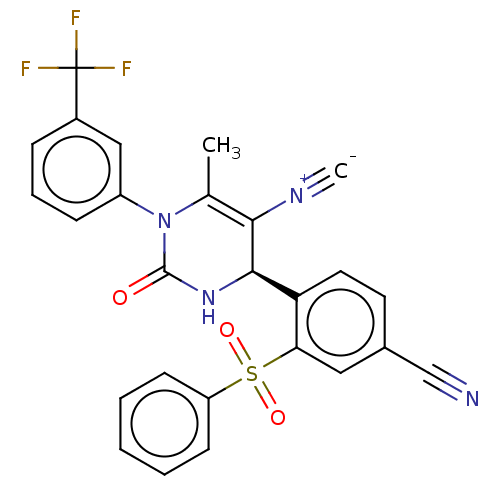 (US9174997, 141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189924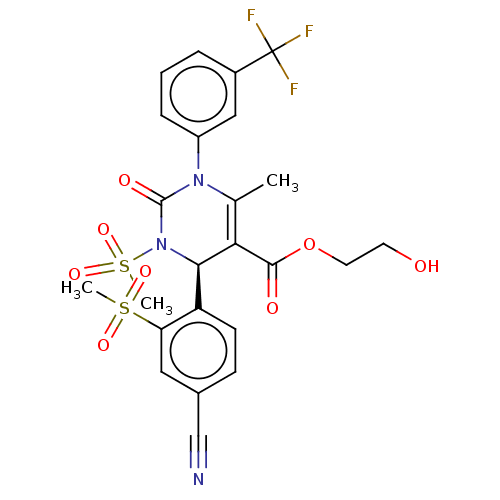 (US9174997, 144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189908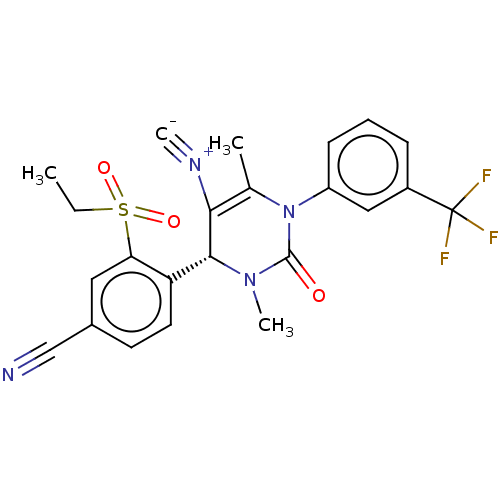 (US9174997, 128) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189900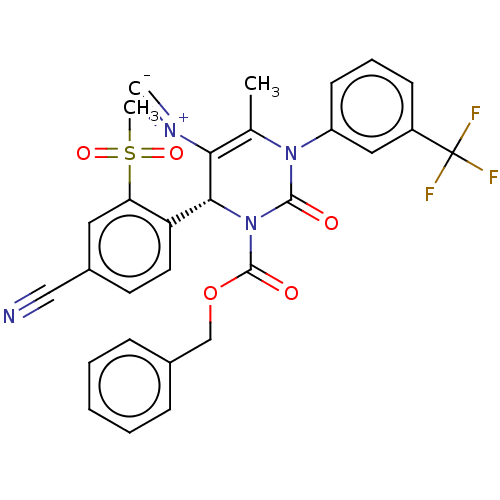 (US9174997, 120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189896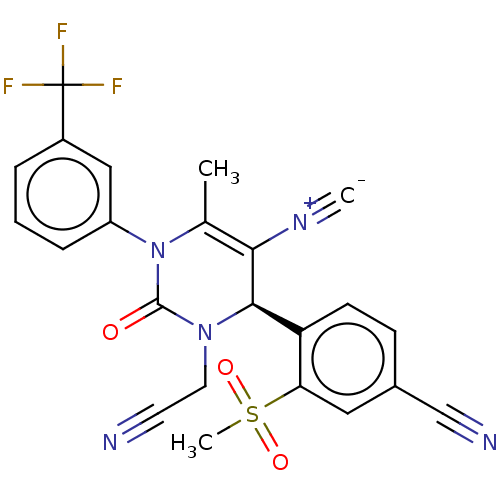 (US9174997, 116) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189876 (US9174997, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189883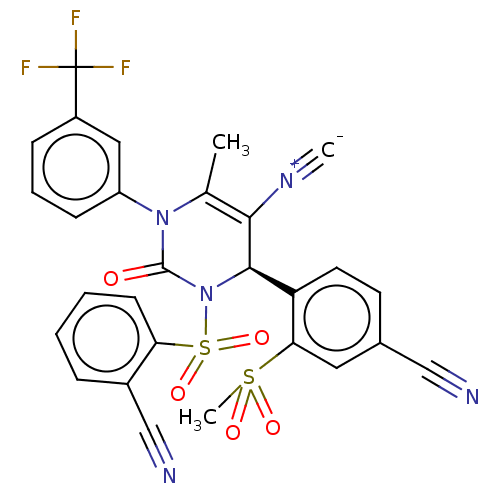 (US9174997, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189871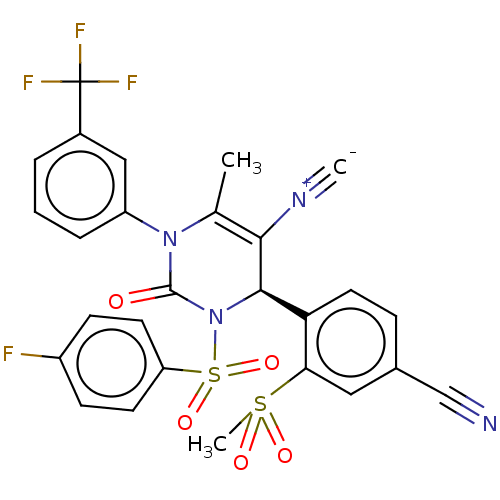 (US9174997, 91) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189865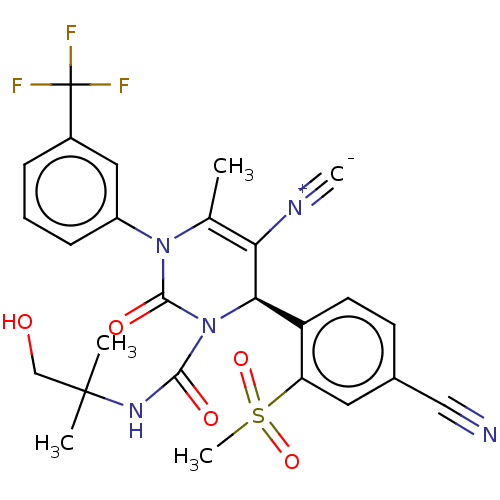 (US9174997, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189860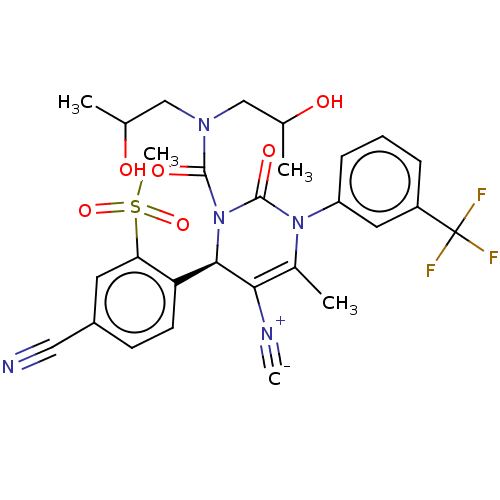 (US9174997, 80) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189835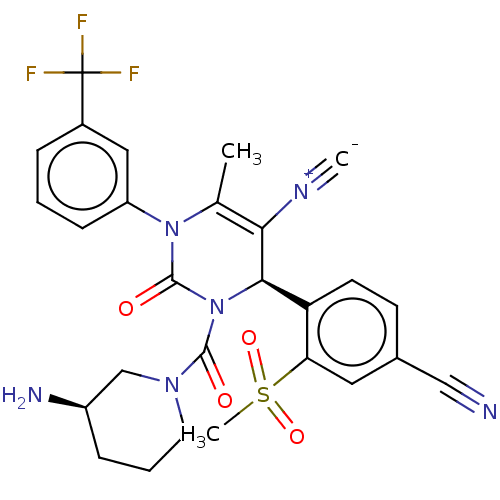 (US9174997, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189831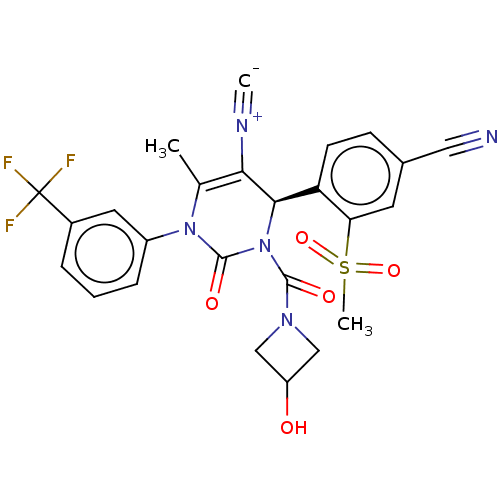 (US9174997, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189823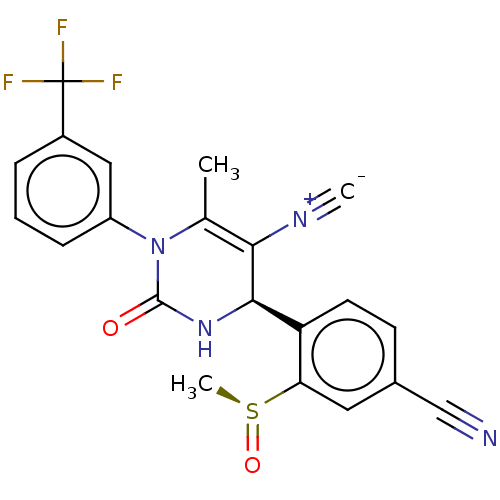 (US9174997, 43 (Diastereomer 1)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189822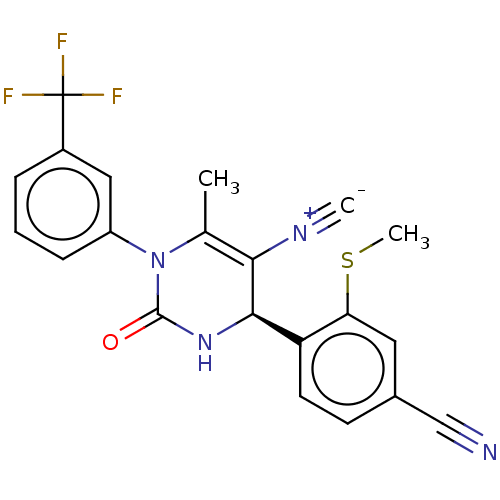 (US9174997, 41) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189821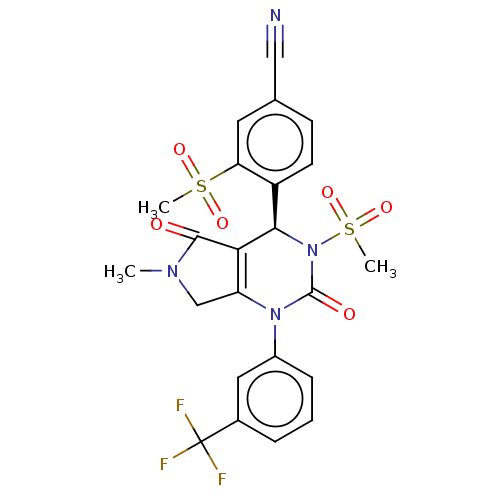 (US9174997, 36) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189820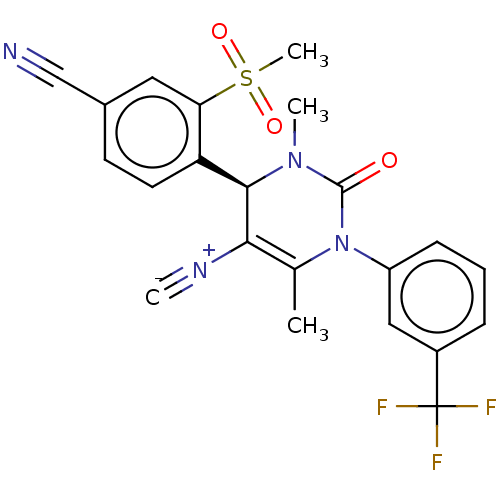 (US9174997, 33) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189819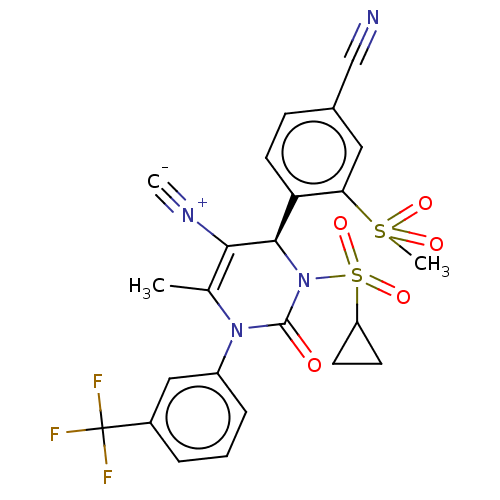 (US9174997, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189818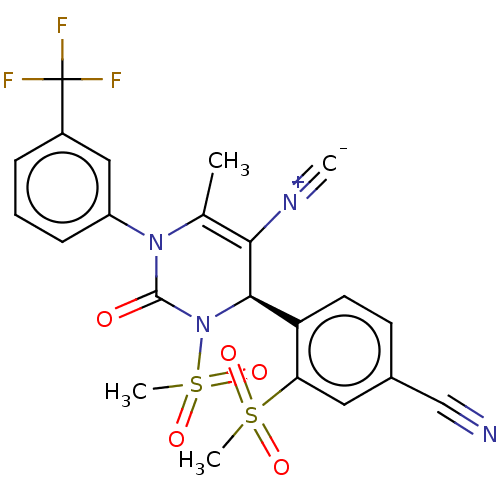 (US9174997, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104820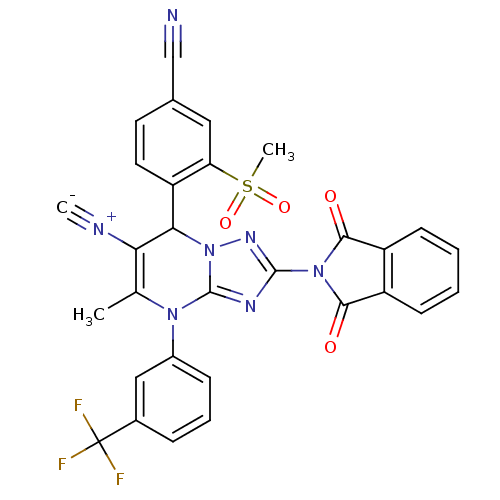 (US8569314, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104821 (US8569314, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104822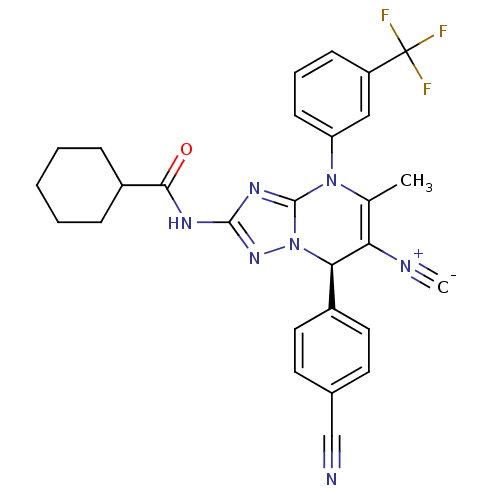 (US8569314, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104823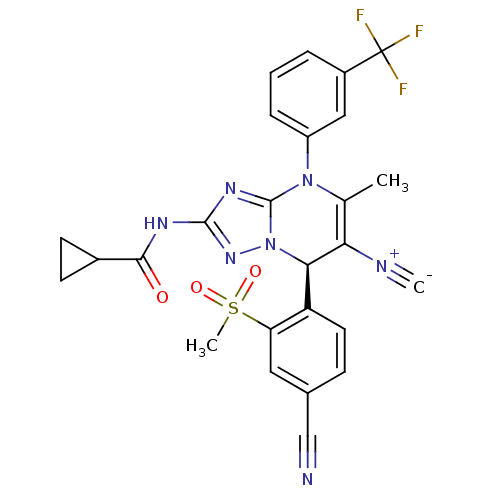 (US8569314, 35) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104824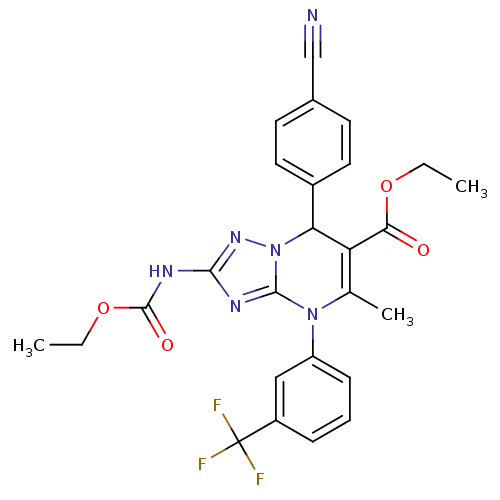 (US8569314, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104825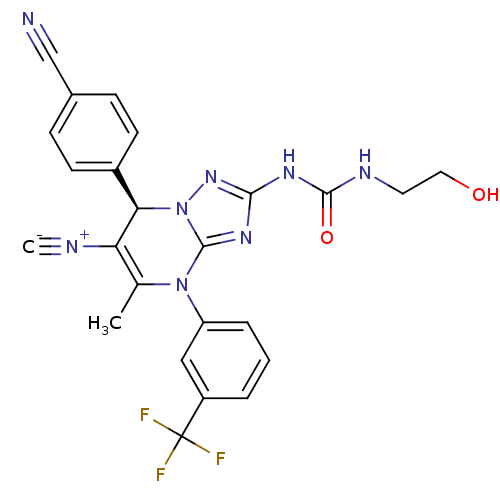 (US8569314, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM104826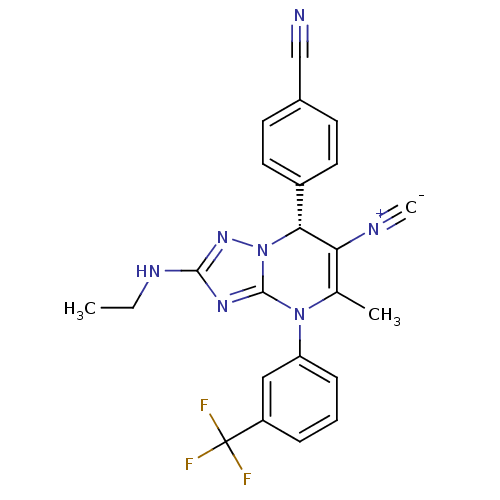 (US8569314, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description In vitro HNE inhibition assay. The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amido... | US Patent US8569314 (2013) BindingDB Entry DOI: 10.7270/Q2FJ2FF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189812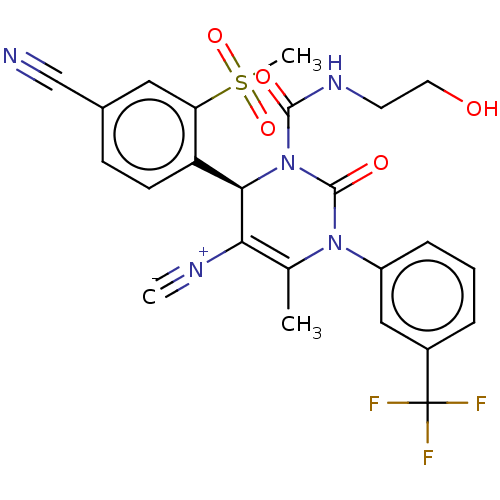 (US9174997, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189813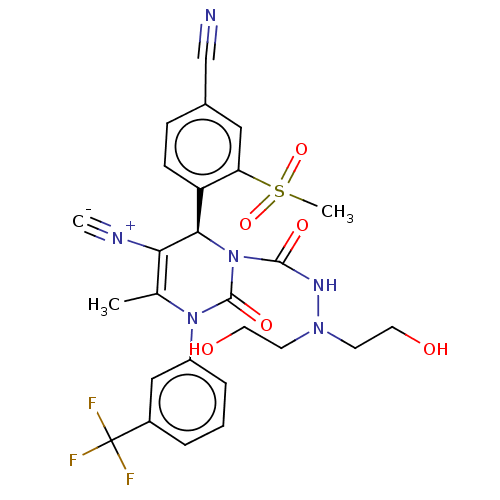 (US9174997, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189814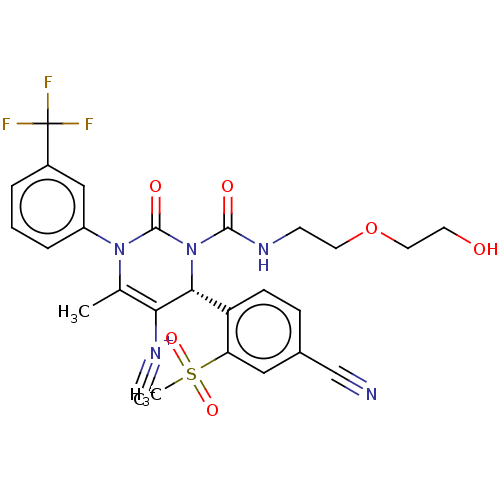 (US9174997, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM189815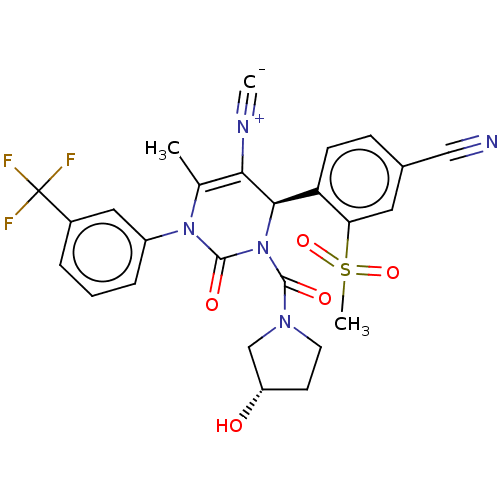 (US9174997, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Bayer Intellectual Property GmbH US Patent | Assay Description The potency of the compounds of the invention is ascertained in an in vitro inhibition assay. The HNE-mediated amidolytic cleavage of a suitable pept... | US Patent US9174997 (2015) BindingDB Entry DOI: 10.7270/Q2GT5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721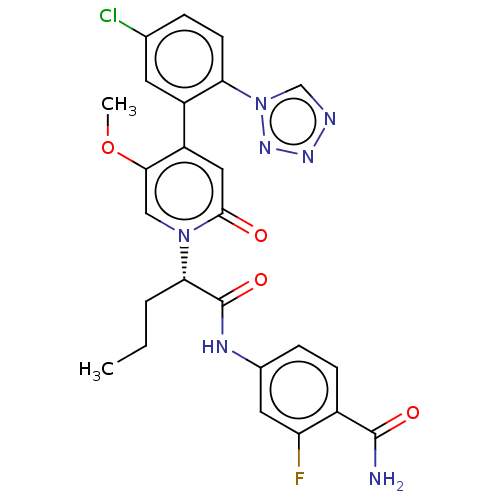 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413721 (4-{[(2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413626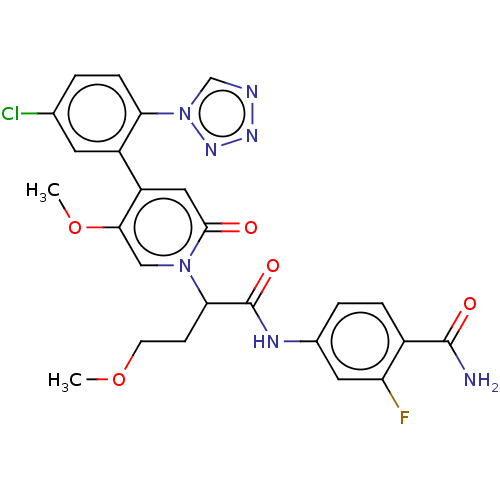 (4-[(2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413727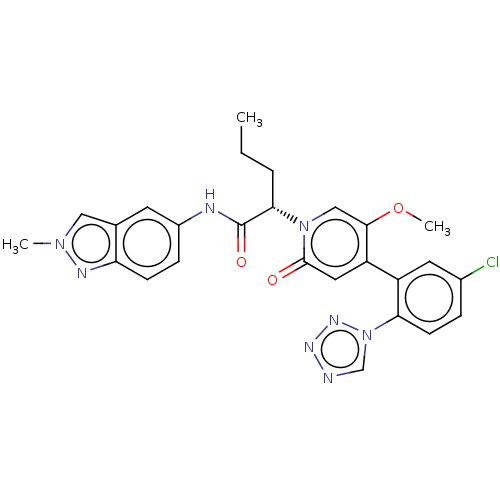 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413626 (4-[(2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413727 ((2S)-2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413719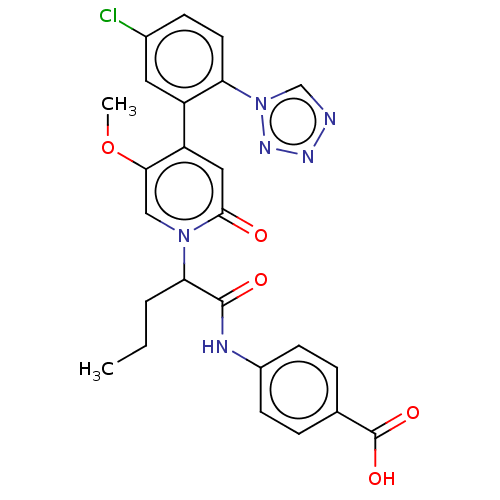 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413654 (4-[(2-{4-[5-Chloro-2-(1,2-oxazol-3-yl)phenyl]-5-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413745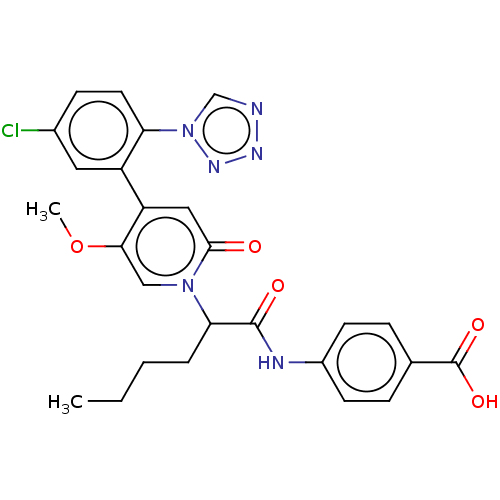 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT US Patent | Assay Description The factor XIa inhibition of the substances according to the invention is determined using a biochemical test system which utilizes the reaction of a... | US Patent US10421742 (2019) BindingDB Entry DOI: 10.7270/Q26M3967 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM413719 (4-{[2-{4-[5-Chloro-2-(1H-tetrazol-1-yl)phenyl]-5-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description FXIa: Test substances are dissolved in dimethyl sulphoxide and serially diluted in dimethyl sulphoxide (3000 μM to 0.0078 μM; resulting fin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2W09935 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1580 total ) | Next | Last >> |